
The particle character of electromagnetic radiation
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص250-252
الجزء والصفحة:
ص250-252
 2025-11-20
2025-11-20
 52
52
The particle character of electromagnetic radiation
The observation that electromagnetic radiation of frequency ν can possess only the energies 0, hν, 2hν, ... suggests that it can be thought of as consisting of 0, 1, 2, ... particles, each particle having an energy hν. Then, if one of these particles is present, the energy is hν, if two are present the energy is 2hν, and so on. These particles of electromagnetic radiation are now called photons. The observation of discrete spectra from atoms and molecules can be pictured as the atom or molecule generating a photon of energy hν when it discards an energy of magnitude ∆E, with ∆E = hν.

Further evidence for the particle-like character of radiation comes from the meas urement of the energies of electrons produced in the photoelectric effect. This effect is the ejection of electrons from metals when they are exposed to ultraviolet radiation. The experimental characteristics of the photoelectric effect are as follows: 1 No electrons are ejected, regardless of the intensity of the radiation, unless its frequency exceeds a threshold value characteristic of the metal. 2 The kinetic energy of the ejected electrons increases linearly with the frequency of the incident radiation but is independent of the intensity of the radiation. 3 Even at low light intensities, electrons are ejected immediately if the frequency is above the threshold.

Fig. 8.13 In the photoelectric effect, it is found that no electrons are ejected when the incident radiation has a frequency below a value characteristic of the metal and, above that value, the kinetic energy of the photoelectrons varies linearly with the frequency of the incident radiation.
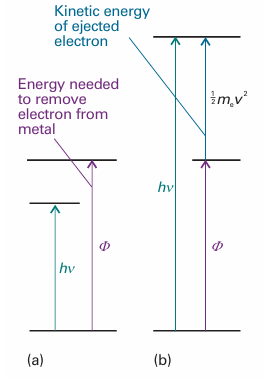
Fig. 8.14 The photoelectric effect can be explained if it is supposed that the incident radiation is composed of photons that have energy proportional to the frequency of the radiation. (a) The energy of the photon is insufficient to drive an electron out of the metal. (b) The energy of the photon is more than enough to eject an electron, and the excess energy is carried away as the kinetic energy of the photoelectron (the ejected electron).
Figure 8.13 illustrates the first and second characteristics. These observations strongly suggest that the photoelectric effect depends on the ejection of an electron when it is involved in a collision with a particle-like projectile that carries enough energy to eject the electron from the metal. If we suppose that the projectile is a photon of energy hν, where ν is the frequency of the radiation, then the conservation of energy requires that the kinetic energy of the ejected electron should obey , 1/2 mev2 =hν−Φ , In this expression Φ is a characteristic of the metal called its work function, the energy required to remove an electron from the metal to infinity (Fig. 8.14), the analogue of the ionization energy of an individual atom or molecule. Photoejection cannot occur if hν < Φ because the photon brings insufficient energy: this conclusion accounts for observation (1). Equation 8.11 predicts that the kinetic energy of an ejected electron should increase linearly with frequency, in agreement with observation (2). When a photon collides with an electron, it gives up all its energy, so we should expect electrons to appear as soon as the collisions begin, provided the photons have sufficient energy; this conclusion agrees with observation (3). A practical application of eqn 8.11 is that it provides a technique for the determination of Planck’s constant, for the slopes of the lines in Fig. 8.13 are all equal to h.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


