
Atomic and molecular spectra
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص248-249
الجزء والصفحة:
ص248-249
 2025-11-20
2025-11-20
 55
55
Atomic and molecular spectra
The most compelling evidence for the quantization of energy comes from spectroscopy, the detection and analysis of the electromagnetic radiation absorbed, emitted, or scattered by a substance. The record of the intensity of light intensity transmitted or scattered by a molecule as a function of frequency (ν), wavelength (λ), or wavenumber (# =ν/c) is called its spectrum (from the Latin word for appearance). A typical atomic spectrum is shown in Fig. 8.10, and a typical molecular spectrum is shown in Fig. 8.11. The obvious feature of both is that radiation is emitted or absorbed at a series of discrete frequencies. This observation can be understood if the energy of the atoms or molecules is also confined to discrete values, for then energy can be discarded or absorbed only in discrete amounts (Fig. 8.12). Then, if the energy of an atom decreases by ∆E, the energy is carried away as radiation of frequency ν, and an emission ‘line’, a sharply defined peak, appears in the spectrum. We say that a molecule undergoes a spectroscopic transition, a change of state, when the Bohr frequency condition
∆E=hν
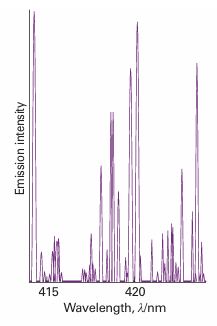
Fig. 8.10 A region of the spectrum of radiation emitted by excited iron atoms consists of radiation at a series of discrete wavelengths (or frequencies).
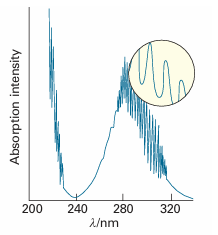
Fig. 8.11 When a molecule changes its state, it does so by absorbing radiation at definite frequencies. This spectrum is part of that due to the electronic, vibrational, and rotational excitation of sulfur dioxide (SO2) molecules. This observation suggests that molecules can possess only discrete energies, not an arbitrary energy.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


