

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Fluxional behaviour of metallocenes
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص562-563
2025-10-04
65
Fluxional behaviour of metallocenes
Key point: Many metallocenes exhibit fluxionality and undergo internal rotation because the barrier to the interconversion of the various forms is low.
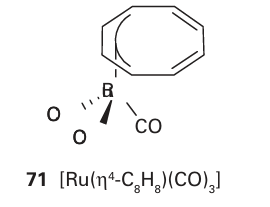
One of the most remarkable aspects of many cyclic polyene complexes is their stereo chemical nonrigidity (their fluxionality). For example, at room temperature the two rings in ferrocene rotate rapidly relative to each other as there is only a low staggered-eclipsed conversion barrier. This type of fluxional process is called internal rotation, and is similar to the process by which the two CH3 groups rotate relative to each other in ethane. We have already noted how, in the gas phase, the eclipsed conformation of ferrocene is slightly more stable than the staggered one; however, the steric bulk of substituents on the metallocene rings can change the barrier and can make staggered the preferred conformation. The rings of metallocenes are often drawn in a staggered conformation simply because there is then a little more space to illustrate substitutions. Of greater interest is the stereochemical nonrigidity that is often seen when a conjugated cyclic polyene is attached to a metal atom through some, but not all, of its C atoms. In such complexes the metal ligand bonding may hop around the ring; in the informal jargon of organometallic chemists this internal rotation is called ‘ring whizzing’. A simple example is found in [Ge( 1-Cp)(CH3)3], in which the single site of attachment of the Ge atom to the cyclopentadiene ring hops around the ring in a series of 1,2-shifts, a motion in which a CM bond is replaced by a CM bond to the next C atom around the ring; this motion is known as a 1,2-shift, because the bond starts on atom 1 and ends up on the adjacent atom 2 (Fig. 22.14). The great majority of fluxional conjugated polyene complexes that have been investigated migrate by 1,2-shifts, but it is not known whether these shifts are controlled by a principle of least motion or by some aspect of orbital symmetry.
Nuclear magnetic resonance provides the primary evidence for the existence and mechanism of these fluxional processes, as they occur on a timescale of 10 2 to 10 4 s, and can be studied by 1H- and 13C-NMR. The compound [Ru(4-C8H8) (CO)3] (71) provides a good illustration of the approach. At room temperature, its 1H-NMR spectrum consists of a single, sharp line that could be interpreted as arising from a symmetrical 8-C8 H8 ligand. However, X-ray diffraction studies of single crystals show unambiguously that the ligand is tetrahapto. This conflict is resolved by 1H-NMR spectra at lower temperatures because as the sample is cooled the signal broadens and then separates into four peaks. These peaks are expected for the four pairs of protons of an 4-C8 H8 ligand. The interpretation is that at room temperature the ring is ‘whizzing’ around the metal atom rapidly compared with the timescale of the NMR experiment, so an averaged signal is observed. At lower temperatures the motion of the ring is slower, and the distinct conformations exist long enough to be resolved. A detailed analysis of the line shape of the NMR spectra can be used to measure the activation energy of the migration.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















