

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Spectroscopic properties of carbonyl compounds
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص558-559
2025-10-04
61
Spectroscopic properties of carbonyl compounds
Key points: The CO stretching frequency is decreased when it serves as a π acceptor; donor ligands cause the CO stretching frequency to decrease as they supply electrons to the metal; 13C-NMR is of less use as many carbonyl compounds are fluxional on the NMR timescale.
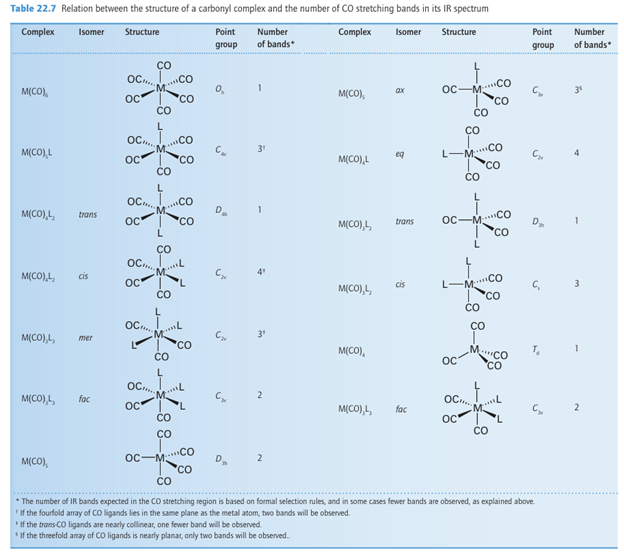
Infrared and 13C-NMR spectroscopy are widely used to determine the arrangement of atoms in metal carbonyl compounds, as separate signals are observed for inequivalent CO ligands. NMR spectra generally contain more detailed structural information than IR spec tra provided the molecule is not fluxional (the timescales of the NMR and IR transition are different, Sections 8.4, 8.5). However, IR spectra are often simpler to obtain, and are particularly useful for following reactions. Most CO stretching bands occur in the range 2100 1700 cm1, a region that is generally free of bands due to organic groups. Both the range of CO stretching frequencies (see Fig. 22.4) and the number of CO bands (Table 22.7) are important for making structural inferences. Group theory allows us to predict the number of active CO stretches in both the IR and Raman spectra (Section 6.5). If the CO ligands are not related by a centre of inversion or a threefold or higher axis of symmetry, a molecule with N CO ligands will have N CO stretching absorption bands. Thus, a bent OCMCO group (with only a twofold symmetry axis) will have two infrared absorptions because both the symmetric (69) and antisymmetric (70) stretches cause the electric dipole moment to change and are IR active. Highly symmetrical molecules have fewer bands than CO ligands. Thus, in a linear OC MCO group, only one IR band (corresponding to the out-of-phase stretching of the two CO ligands) is observed in the CO stretching region because the symmetrical stretch leaves the overall electric dipole moment unchanged. As shown in Fig. 22.12, the positions of the CO ligands in a metal carbonyl may be more symmetrical than the point group of the whole compound suggests and then fewer bands will be observed than are predicted on the basis of the overall point group. Raman spectroscopy can be very useful in assign ing structures because the selection rules complement those of IR (Sections 6.5 and 8.4). Thus, for a linear OCMCO group, the symmetrical stretching of the two CO ligands is observed in the Raman spectrum. As we noted in Section 22.5, infrared spectroscopy is also useful for distinguishing terminal CO (MCO) from bridging CO (μ2-CO) and face-bridging CO (μ3-CO). It can also be used to determine the order of π-acceptor strengths for the other ligands present in a complex. A motionally averaged NMR signal is observed when a molecule undergoes changes in structure more rapidly than the technique can resolve (Section 8.5). Although this phenomenon is quite common in the NMR spectra of organometallic compounds, it is not normally observed in their IR or Raman spectra. An example of this difference is Fe (CO)5,
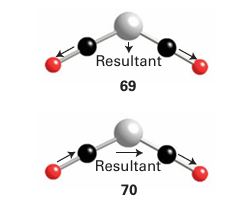
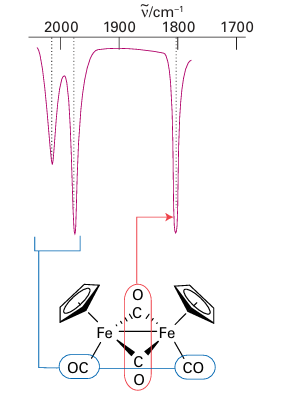
Figure 22.12 The infrared spectrum of [Fe2 (Cp)2(CO)4]. Note the two high-frequency terminal CO stretches and the lower frequency absorption of the bridging CO ligands. Although two bridging CO bands would be expected on account of the low symmetry of the complex, a single band is observed because the two bridging CO groups are nearly collinear.
for which the 13C-NMR signal shows a single line at Ϭ=210, whereas IR and Raman spectra are consistent with a trigonal-bipyramidal structure.


 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















