

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Magnetic measurements
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص478-479
2025-09-24
53
Magnetic measurements
Key points: Magnetic measurements are used to determine the number of unpaired spins in a complex and hence to identify its ground-state configuration. A spin-only calculation may fail for low-spin d5 and for high-spin 3d6 and 3d7 complexes. The experimental distinction between high-spin and low-spin octahedral complexes is based on the determination of their magnetic properties. Compounds are classified as diamagnetic if they are repelled by a magnetic field and paramagnetic if they are attracted by a magnetic field. The two classes are distinguished experimentally by magnetometry. The magnitude of the paramagnetism of a complex is commonly reported in terms of the magnetic dipole moment it possesses: the higher the magnetic dipole moment of the complex, the greater the paramagnetism of the sample. In a free atom or ion, both the orbital and the spin angular momenta give rise to a magnetic moment and contribute to the paramagnetism. When the atom or ion is part of a complex, any orbital angular momentum is normally quenched, or suppressed, as a result of the interactions of the electrons with their nonspherical environment. However, if any electrons are unpaired the net electron spin angular momentum survives and gives rise to spin-only paramagnetism, which is characteristic of many d-metal complexes. The spin only magnetic moment, μ, of a complex with total spin quantum number S is
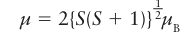
Where μB is the Bohr magneton, μB= eh/2me with the value 9.274 10 24 J T1. Because S=1/2 N, where N is the number of unpaired electrons, each with spin s=1/2,
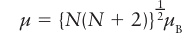
Ameasurement of the magnetic moment of a d-block complex can usually be interpreted in terms of the number of unpaired electrons it contains, and hence the measurement can be used to distinguish between high-spin and low-spin complexes. For example, magnetic measurements on a d6 complex easily distinguish between a high-spin t2g4 eg2 (N=4, S=2, μ=4.90μB) configuration and a low-spin t2g 6 (N=0, S=0, μ=0) configuration. The spin-only magnetic moments for some electron configurations are listed in Table 20.3 and compared there with experimental values for a number of 3d complexes. For most 3d complexes (and some 4d complexes), experimental values lie reasonably close to spin-only predictions, so it becomes possible to identify correctly the number of unpaired electrons and assign the ground-state configuration. For instance, [Fe (OH2)6]3+ is paramagnetic with a magnetic moment of 5.9μB. As shown in Table 20.3, this value is consistent with there be ing five unpaired electrons (N=5 and S=5/2 ), which implies a high-spin t2g3 eg2 configuration.
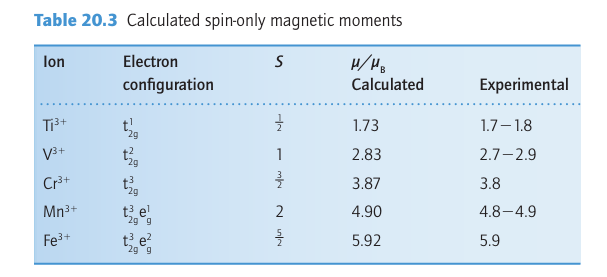
The interpretation of magnetic measurements is sometimes less straightforward than this example might suggest. For example, the potassium salt of [Fe(CN)6]3- has μ=2.3μB, which is between the spin-only values for one and two unpaired electrons (1.7μB and 2.8μB , respectively). In this case, the spin-only assumption has failed because the orbital contribution to the magnetic moment is substantial.
For orbital angular momentum to contribute, and hence for the paramagnetism to dif fer significantly from the spin-only value, there must be one or more unfilled or half-filled orbitals similar in energy to the orbitals occupied by the unpaired spins and of the appropriate symmetry (one that is related to the occupied orbital by rotation round the diretion of the applied field). If that is so, the applied magnetic field can force the electrons to circulate around the metal ion by using the low-lying orbitals and hence it generates orbital angular momentum and a corresponding orbital contribution to the total magnetic moment (Fig. 20.6). Departure from spin-only values is generally large for low-spin d5 and for high-spin 3d6 and 3d7 complexes. It is also possible for the electronic state of the metal ion to change (for example with temperature), leading to a change from high-spin to low-spin and a change in the magnetic moment. Such complexes are referred to as spin-crossover complexes and are discussed in more detail, together with the effects of cooperative magnetism, in Sections 20.8 and 20.9.
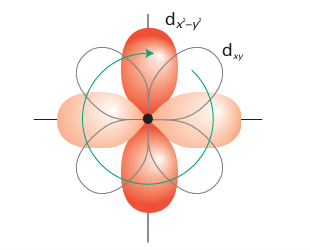
Figure 20.6 If there is a low-lying orbital of the correct symmetry, the applied field may induce the circulation of the electrons in a complex and hence generate orbital angular momentum. This diagram shows the way in which circulation may arise when the field is applied perpendicular to the xy-plane (perpendicular to this page).

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















