

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Octahedral complexes
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
473-475
2025-09-24
56
Octahedral complexes
Key points: In the presence of an octahedral crystal field, d orbitals are split into a lower-energy triply degenerate set (t2g) and a higher-energy doubly degenerate set (eg) separated by an energy ΔO; the ligand-field splitting parameter increases along a spectrochemical series of ligands and varies with the identity and charge of the metal atom. In the model of an octahedral complex used in crystal-field theory, six-point negative charges representing the ligands are placed in an octahedral array around the central metal ion. These charges (which we shall refer to as the ‘ligands’) interact strongly with the
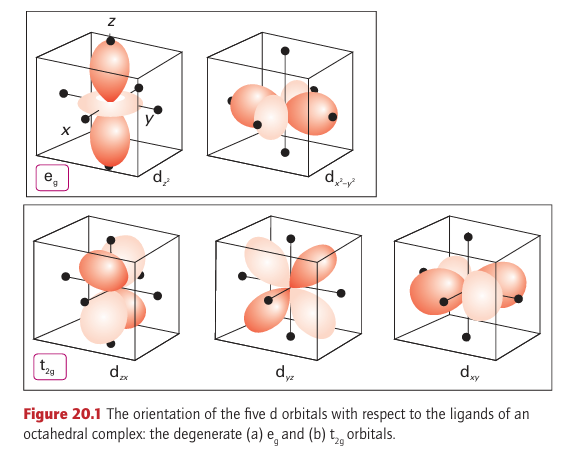
central metal ion, and the stability of the complex stems in large part from this attractive interaction between opposite charges. However, there is a much smaller but very important secondary effect arising from the fact that electrons in different d orbitals interact with the ligands to different extents. Although this differential interaction is little more than about 10 per cent of the overall metal ligand interaction energy, it has major consequences for the properties of the complex and is the principal focus of this section. Electrons in dz2 and dx2 y2 orbitals (which are of symmetry type eg in Oh; Section 6.1) are concentrated close to the ligands, along the axes, whereas electrons in dxy, dyz, and dzx orbitals (which are of symmetry type t2g ) are concentrated in regions that lie between the ligands (Fig. 20.1). As a result, the former are repelled more strongly by the negative charge on the ligands than the latter and lie at a higher energy. Group theory shows that the two eg orbitals have the same energy (although this is not readily apparent from drawings), and that the three t2g orbitals also have the same energy. This simple model leads to an energy-level diagram in which the three degenerate t2g orbitals lie below the two degenerate eg orbitals (Fig. 20.2). The separation of the two sets of orbitals is called the ligand-field splitting parameter, O (where the subscript O signifies an octahedral crystal field).
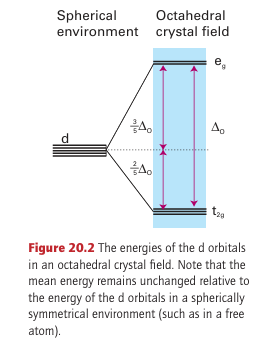
The energy level that corresponds to the hypothetical spherically symmetrical environ ment (in which the negative charge due to the ligands is evenly distributed over a sphere instead of being localized at six points) defines the barycentre of the array of levels, with the two eg orbitals lying at 3/5 ∆O above the barycentre and the three t2g orbitals lying at 2/5 ∆O below it. As in the representation of the configurations of atoms, a superscript is used to indicate the number of electrons in each set, for example t2g2.
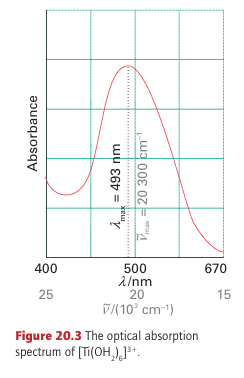
The simplest property that can be interpreted by crystal-field theory is the absorption spectrum of a one-electron complex. Figure 20.3 shows the optical absorption spectrum of the d1 hexaaquatitanium (III) ion, [Ti(OH2)6]3+. Crystal-field theory assigns the first absorption maximum at 493 nm (20300 cm-1) to the transition eg ← t2g and identifies 20300cm1 with ∆O for the complex. It is not so straightforward to obtain values of ∆O for complexes with more than one d electron because the energy of a transition then depends not only on orbital energies but also on the electron-electron repulsion energies. This aspect is treated more fully in Section 20.4 and the results from the analyses described there have been used to obtain the values of ∆O in Table 20.1.
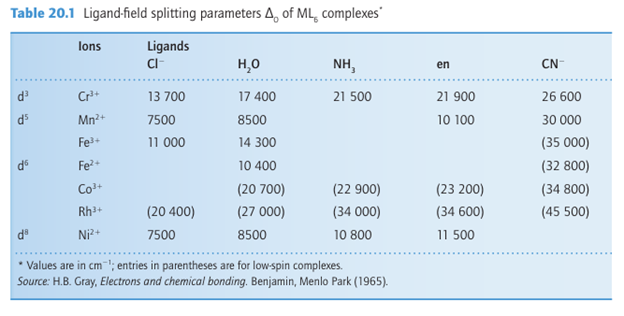
The ligand-field splitting parameter, ∆O , varies systematically with the identity of the lig and. For instance, in the series of complexes [CoX(NH3)5]n with X I, Br, Cl, H2O, and NH3, the colours range from purple (for X I ) through pink (for Cl) to yellow (with NH3 ). This sequence indicates that the energy of the lowest energy electronic transition (and therefore ∆O) increases as the ligands are varied along the series. The same order is followed regardless of the identity of the metal ion. Thus ligands can be arranged in a spectrochemical series, in which the members are arranged in order of increasing energy of transitions that occur when they are present in a complex:
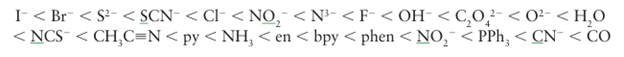
(The donor atom in an ambidentate ligand is underlined.) Thus, the series indicates that, for the same metal, the optical absorption of the cyano complex will occur at higher en ergy than that of the corresponding chlorido complex. A ligand that gives rise to a high energy transition (such as CO) is referred to as a strong-field ligand, whereas one that gives rise to a low-energy transition (such as Br-) is referred to as a weak-field ligand. Crystal field theory alone cannot explain these strengths, but ligand-field theory can, as we shall see in Section 20.2. The ligand-field strength also depends on the identity of the central metal ion, the order being approximately:
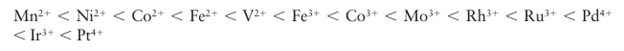
The value of ∆O increases with increasing oxidation state of the central metal ion (compare the two entries for Fe and Co) and also increases down a group (compare, for instance, the locations of Co, Rh, and Ir). The variation with oxidation state reflects the smaller size of more highly charged ions and the consequently shorter metal ligand distances and stronger interaction energies. The increase down a group reflects the larger size of the 4d and 5d orbitals compared with the compact 3d orbitals and the consequent stronger interactions with the ligands.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















