

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Intermediate oxidation states in the 3d series
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص454-456
2025-09-23
84
Intermediate oxidation states in the 3d series
Key point: Oxidation state +3 is common to the left of the 3d series and +2 is common for metals from the middle to the right of the block.
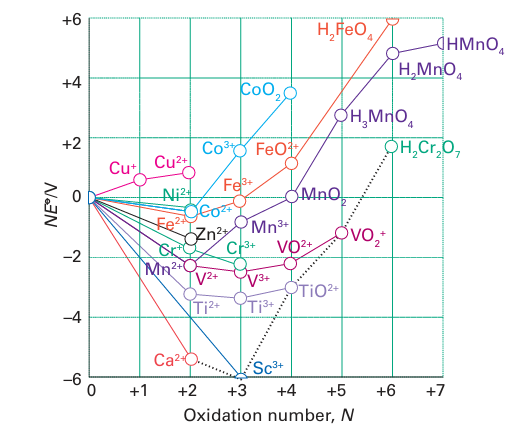
Figure 19.3 A Frost diagram for the first series of the d-block elements in acidic solution (pH=0). The broken line connects species in their group oxidation states.
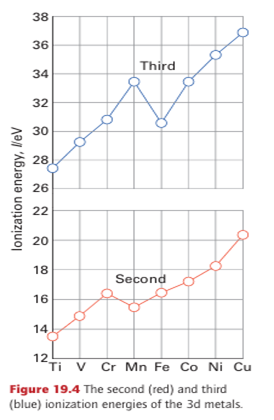
Most unipositive d-metal ions (M) disproportionate (to M and M+2) because the bonds in the solid metal are so strong and, for the 3d metals, the+ 2 oxidation state is generally the lowest one it is necessary to consider in aqueous solution. Exceptions are mainly confined to metal-metal bonded and organometallic compounds, such as the metal carbonyls Ni (CO)4 and Mo (CO)6 discussed in Chapter 22, in which the metal is in oxidation state 0. Figure 19.4 shows the second and third ionization energies of the 3d metals, and we can see the expected increase across the period, in line with increasing nuclear charge. The anomalous values for manganese and iron are a result of the very stable d5 configurations of the Mn2 and Fe3 ions. The dipositive aqua ions, M2 (aq) (specifically, the octahedral complexes [M(OH2)6 ]+2), play an important role in the chemistry of the 3d-series metals. Many of these ions are coloured as a result of d-d transitions in the visible region of the spectrum (Section 20.4). For example, Cr2+ (aq) is blue, Fe2+ (aq) is green, Co2+ (aq) is pink, Ni2+ (aq) is green, and Cu2+ (aq) is blue.
The +3-oxidation state is common at the left of the period, and is the only oxidation state normally encountered for scandium. Careful inspection of the Frost diagrams in Fig. 19.3 gives the balance of stability between the 3 and 2 oxidation states. Titanium, vanadium, and chromium all form a wide range of compounds in oxidation state 3, and under normal conditions the 3-oxidation state is more stable than the +2 state. Manganese (II) is especially stable due to its half-filled d shell, and relatively few Mn (III) compounds are known. Beyond manganese, many Fe (III) complexes are known, but are often oxidizing. In acid solution, Co3+ (aq) is powerfully oxidizing and O2 is evolved:
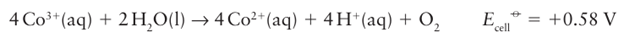
Species such as Co (III) are stabilized as oxido compounds (for instance, CoO(OH)), which are formed under basic conditions (Section 19.8), or when complexed by good donor ligands such as amines. Aqua ions of Ni3 and Cu3 have not been prepared. In contrast, M(II) becomes increasingly common from left to right across the series. For example, among the early members of the 3d series, Sc2+ (aq) (Group 3) is unknown and Ti2+ (aq) (Group 4) is formed only upon bombarding solutions of Ti3+ with electrons in the technique known as pulse radiolysis. For Groups 5 and 6, V2+ (aq) and Cr2+ (aq) are thermodynamically unstable with respect to oxidation by H ions:
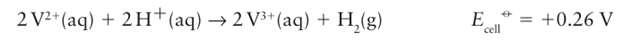
Despite this instability, the slowness of the evolution of H2 makes it possible to work with aqueous solutions of these dipositive ions in the absence of air, and as a result they are useful reducing agents. Beyond Cr (for Mn2, Fe2, Co2+, Ni2+, and Cu2+), M(II) is stable with respect to reaction with water, and only Fe2 is oxidized by air. Water is incompatible with many metal ions because it can serve as an oxidizing agent. A wider range of M2+ ions is therefore known in solids than in aqueous solution. For example, TiCl2+ can be prepared by the reduction of TiCl4 with hexamethyldisilane,
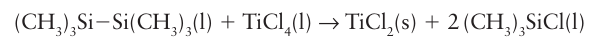
but it is oxidized by both water and air to give TiO2. With the exception of ScCl2, these dihalides are well described by structures containing isolated M2+ ions (Table 19.2). The fluorides have a rutile structure (Section 3.9) and most of the heavier halides are layered;
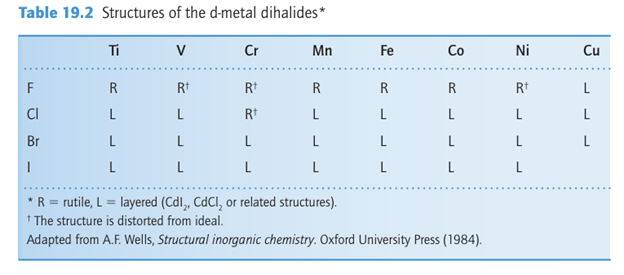
in both cases the metal is in an octahedral site. This shift in structure is understandable because the rutile structure type is associated with ionic bonding and the layered structures are associated with more covalent bonding.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















