

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Polyoxometallates
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص462-463
2025-09-24
54
Polyoxometallates
Key points: In their highest oxidation states, metals in Groups 5 and 6 readily form polyoxometallates and heteropoly oxometallates; pH is crucial in determining which compounds form. Apolyoxometallate is an oxoanion containing more than one metal atom. The H2O ligand created by protonation of an oxido ligand at low pH can be eliminated from the central metal atom and thus lead to the condensation of mononuclear oxometallates. A familiar example is the reaction of a basic chromate solution, which is yellow, with excess acid to form the oxido-bridged dichromate ion, which is orange:
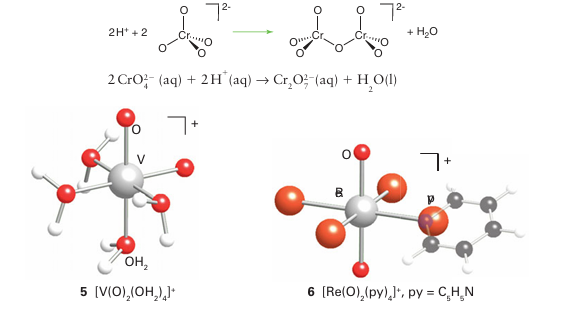
In highly acidic solution, oxido-bridged Cr (VI) species with longer chains are formed. The tendency for Cr (VI) to form polyoxo species is limited by the fact that the O tetrahedra link only through vertices: edge and face bridging would result in too close an approach of the metal atoms. By contrast, it is found that five- and six-coordinate metal oxido complexes, which are common with the larger 4d- and 5d-series metal atoms, can share oxido ligands between either vertices or edges. These structural possibilities lead to a richer variety of polyoxometallates than found with the 3d series metals. Chromium’s neighbours in Groups 5 and 6 form six-coordinate polyoxo complexes (Fig. 19.12). In Group 5, the polyoxometallates are most numerous for vanadium, which forms many V(V) complexes and a few V(IV) or mixed oxidation state V(IV) V(V) poly oxido complexes. Polyoxometallate formation is most pronounced in Groups 5 and 6 for V(V), Mo (VI), and W(VI).
It is often convenient to represent the structures of the polyoxometallate ions by poly hedra, with the metal atom understood to be in the centre and O atoms at the vertices. For example, the sharing of O atom vertices in the dichromate ion, Cr2O72, may be depicted in either the traditional way (7) or in the polyhedral representation (8). Similarly, the important M6 O19 structure of [Nb6O19 ]8- , [Ta6O19 ]8-, [Mo6O19 ]2- , and [W6O19 ]2- is depicted by the conventional or polyhedral structures shown in Fig. 19.13. The structures for this series of polyoxometallates contain terminal O atoms (those projecting out from a single metal atom) and two types of bridging O atoms: two-metal bridges, MOM, and one hypercoordinated O atom in the centre of the structure that is common to all six metal atoms. The structure consists of six MO6 octahedra, each sharing an edge with four neighbours. The overall symmetry of the M6O19 array is Oh. Another example of a polyoxometallate is [W12O40 (OH)2]10- (9). As shown by this formula, the polyoxoanion is partially protonated. Polyoxometallate anions can be prepared by carefully adjusting pH and concentrations, for example polyoxomolybdates and polyoxotungstates are formed by acidification of solutions of the simple molybdate or tungstate:
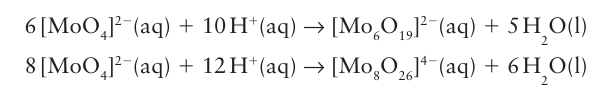
Mixed metal polyoxometallates are also common, as in MoV9O285- , and there is a large class of heteropoly oxometallates, such as the molybdates and tungstates, that also in corporate P, As, and other heteroatoms. For example, [PMo12 O40 ]3 contains a PO4 3 tetrahedron that shares O atoms with surrounding octahedral MoO6 groups (10). Many different heteroatoms can be incorporated into this structure, and the general formula tion is [X(+N)Mo12O40 ](8-N) where X(+N) represents the oxidation state of the hetero atom X, which may be As(V), Si(IV), Ge(IV), or Ti(IV). An even broader range of heteroatoms is observed with the analogous tungsten heteropolyoxoanions. Heteropoly- oxomolybdates and tungstates can undergo one-electron reduction with no change in structure but with the formation of a deep blue colour. The colour seems to arise from the excitation of the added electron from Mo(V) or W(V) to an adjacent Mo(VI) or W(VI) site.
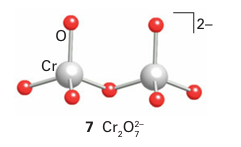
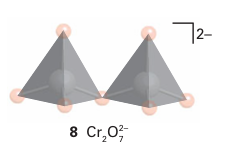
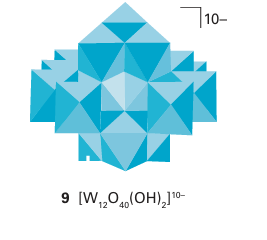
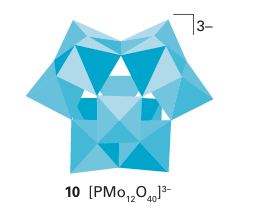
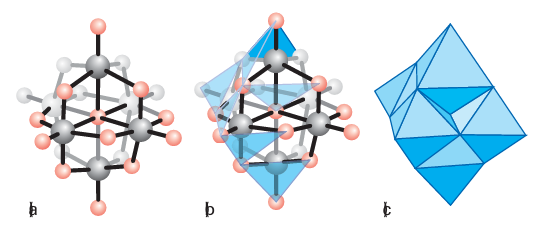
Figure 19.13 (a) Conventional and (c) polyhedral representation of the six edge-shared octahedra as found in [M6O19 ]2-. The intermediate structure (b) shows the process of constructing the polyhedral representation in progress.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















