

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Mononuclear oxido complexes
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص461-462
2025-09-24
52
Mononuclear oxido complexes
Key points: The conversion of an aqua ligand to an oxido ligand is favoured by a high pH and by a high oxidation state of the central metal atom; in vanadium complexes in oxidation state 4 or 5, the site trans to the oxido ligand may be vacant or occupied by a weakly coordinating ligand. Our focus in this section is on the oxido ligand (O-2) and its ability to bring out the high oxidation states of the chemically hard metals on the left of the d block. Another important issue is the relation between oxido and aqua (H2O) ligands resulting from proton transfer equilibria. Elements in high oxidation states typically occur as oxoanions in aqueous solution, such as MnO4, which contains Mn (VII), and CrO4-2, which contains Cr (VI). The existence of these oxoanions contrasts with the existence of simple aqua ions for the same metals in lower oxidation states, such as [Mn (OH2)6]2 for manganese (II) and [Cr (OH2)6 ]3 for chromium (III). The formation of an oxido complex rather than an aqua complex is favoured by high pH because the OH ions in basic solution tend to remove protons from the aqua ligands. The occurrence of aqua complexes for low oxidation state metal cations is explained by noting the relatively small electron-withdrawing effect exerted by these cations on the O atoms of the OH2 ligand. As a result, the aqua ligands are only weak proton donors. A metal ion in a high oxidation state, however, depletes the electron density on the O atoms attached to it and thereby increases the Brønsted acidity of H2O and OH ligands. The influence of pH on the stabilities of different oxidation states is best summarized by a Pourbaix diagram (Section 5.14). The example shown in Fig. 19.9 is for a complex (3) containing a tetradentate ligand L, which results in a similar coordination geometry for a wide range of conditions. The aqua complex cis-[RuLCl(OH2)] in solution at pH 2 is stable up to 0.40 V. Just above this potential, simple oxidation (that is, electron removal) occurs to give cis-[RuLCl(OH2)]2. Under even more strongly oxidizing conditions (at 0.95 V) and pH 2, both oxidation and deprotonation occur and result in the formation of a Ru(IV) oxido species, cis-[RuLCl(O)]. At an even higher potential (about 1.4 V), further oxidation yields cis-[RuLCl(O)]2. As already remarked, the influence of more basic conditions is to deprotonate an OH2 ligand and thus to favour hydroxido or oxido complexes. For example, at pH8, the Ru (III) species is deprotonated to the hydroxido complex cis-[RuLCl(OH)] , which in turn is converted to the oxido-Ru(IV) complex at a lower potential than for the Ru(III) Ru(IV) transformation at pH2. Table 19.5 gives a list of simple complexes containing oxido ligands. Many complexes are known that contain the vanadium (IV) group, VO2 (sometimes referred to as vanadyl), with V in its penultimate oxidation state ( 4). Vanadyl complexes generally contain four additional ligands and are square pyramidal (4). Many of these d1 complexes are blue (as a result of a d-d transition) and may take on a weakly bound sixth ligand trans to the oxido ligand. The vanadyl VO bond length (158 pm) in [VO(acac)2] is short compared with the four VO bond lengths to the acac ligand (197 pm). The short bond lengths in vanadyl complexes, together with high VO stretching wavenumbers (940 1000 cm1) provide strong evidence for VO multiple bonding in which lone pairs on the oxido ligand are donated to the central V atom. The V3d O2p -orbital overlap involved in a multiple bond is illustrated in Fig. 19.10; thus, it can be seen that the O2 ligand is not only a donor but also a donor capable of making two bonds and resulting in a bond order of up to three (though by convention such interactions are depicted as MO, preserving electroneutrality). This strong
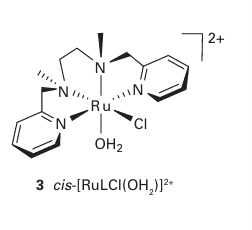
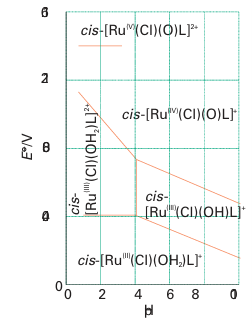
Figure 19.9 A Pourbaix diagram for cis-[RuLCl(OH2)]2 (3) and related species. (Adapted from C.-K. Li, W.-T. Wang, C.-M. Chi, K.-Y. Wong, R.-J. Wang, and T.C.W. Mak, J. Chem. Soc., Dalton Trans. 1991, 1909.)
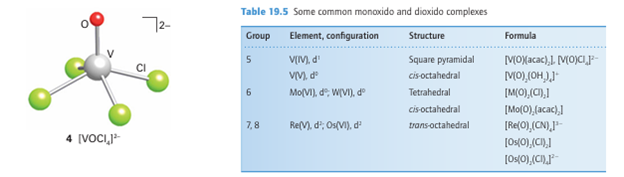
multiple bonding with the O atom appears to be responsible for the trans influence of the oxido ligand, which disfavours attachment of the ligand trans to O (Section 21.4). Vanadium in its highest (and group) oxidation state, 5, forms an extensive series of oxido compounds, many of which are the polyoxo species discussed later. The simplest oxido complex [V(O)2 (OH2)4] exists in the acidic solution formed when the sparingly soluble vanadium(V) oxide, V2O5, dissolves in water. This paleyellow complex has a cis geometry (5). Here again we see the trans influence of an oxido ligand: the cis geometry minimizes competition between the oxido ligands for -bonding to the V atom. As shown in Fig. 19.11, the two O2 ligands in the trans configuration can -bond with the same two d orbitals; in the cis configuration the metal atom has only one d orbital in common with the orbitals on the O atoms. In contrast to the cis structure of the d0 complex [V(O)2 (OH2)4] , many trans-dioxido complexes are known for the d2 metal centres Os(VI) and Re(V) (6); Table 19.5 gives some examples. This geometry is probably favoured because the trans configuration leaves a vacant low-energy orbital that can be occupied by the two d electrons. According to this explanation, the avoidance of the destabilizing effect of the d2 electrons more than offsets the disadvantage of the ligands having to compete for the same d orbital in the trans-oxido geometry.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















