

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Oxidation states
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص264-265
2025-09-03
47
Oxidation states
Key points: The group oxidation number can be predicted from the electron configuration of an element. The inert pair effect leads to an increasing stability of an oxidation state that is 2 less than the group oxidation number for the heavier elements. d-Block elements exhibit a variety of oxidation states. The trends in stable oxidation states within the periodic table can be understood to some extent by considering electron configurations. Related factors such as ionization energies and spin correlation also play a role. A complete or half-full valence shell imparts greater stability than a partially filled shell. Therefore, there is a tendency for atoms to gain or lose electrons until they acquire that configuration. A noble-gas configuration is achieved in the s and p blocks when eight electrons occupy the s and p subshells of the valence shell. In Groups 1, 2, and 13 the loss of electrons to leave the inner complete shell can be achieved with a relatively small input of energy. Thus, for these elements the oxidation numbers typical of the groups are +1, +2, and +3, respectively. From Group 14 to Group 17 it becomes increasingly energetically favour able—provided we consider the overall contributions to the energy, such as the interaction between oppositely charged ions—for the atoms to accept electrons in order to complete the valence shell. Consequently, the group oxidation numbers are -4, -3, -2, -1 with more electronegative elements. Group 18 elements already have a complete octet of electrons and are neither readily oxidized nor reduced. The heavier elements of the p block also form compounds with the element with an oxidation number 2 less than the group oxidation number. The relative stability of an oxidation state in which the oxidation number is 2 less than the group oxidation number is an example of the inert pair effect and it is a recurring theme within the p block. For example, in Group 13 whereas the group oxidation number is +3, the +1 oxidation state increases in stability down the group. In fact, the most common oxidation state of thallium is Tl(I). There is no simple explanation for this effect: it is often ascribed to the large energy that is needed to remove the ns2 electrons after the np1 electron has been removed. However, the sum of the first three ionization energies of Tl (5438 kJ mol -1) is not higher than the value for Ga (5521 kJ mol -1) and only slightly higher than the value for in (5083 kJ mol -1). Another contribution to the effect may be the low M X bond enthalpies for the heavier p-block elements and the decreasing lattice energy as the atomic radii increase down a group.
The group oxidation number is achieved in the d block only for Groups 3 to 8 and even then, highly oxidizing F or O are required to bring it out. For Group 7 and 8, only O can produce anions or neutral oxides with an element with oxidation numbers 7 and 8, as in the permanganate anion, MnO-4 and osmium tetraoxide, OsO4. The range of observed oxidation states is shown in Table 9.4. As can be seen, up to Mn, all the 3d and 4s electrons can participate in bonding and the maximum oxidation state corresponds to the group number. Once the d5 electron configuration is exceeded the tendency for the d electrons to participate in bonding decreases and high oxidation states are not observed. Similar trends exist for the 4d and 5d series. However, the stability of high oxidation states increases down a group in Groups 4 to 10 as the atomic radius increases. The relative stabilities of oxidation states of the 4d and 5d series members of each group are similar as their
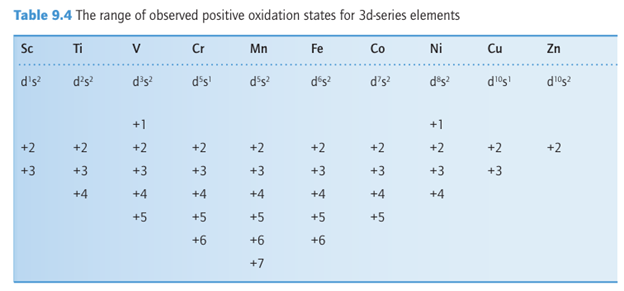
atomic radii are very similar (due to the lanthanide contraction). As already remarked, half-filled shells of electrons with parallel spins are particularly stable due to spin correlation (Section 1.7a). This additional stability has important consequences for the chemistry of the d-block elements with exactly half-filled shells. Manganese has the configuration 3d54s2; as a result, Mn (II) is particularly stable and Mn (III) compounds are uncommon. The importance of spin correlation diminishes as the orbitals become larger, for example Tc and Re, which are the 4d and 5d counterparts of Mn, do not form M(II) compounds. d-Block elements also form stable compounds with the metal in its zero-oxidation state. These complexes are typically stabilized by a ligand that acts as a π-acid.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















