
Pressure
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص3-4
الجزء والصفحة:
ص3-4
 2025-10-29
2025-10-29
 37
37
Pressure
Pressure is defined as force divided by the area to which the force is applied. The greater the force acting on a given area, the greater the pressure. The origin of the force exerted by a gas is the incessant battering of the molecules on the walls of its container. The collisions are so numerous that they exert an effectively steady force, which is experienced as a steady pressure. The SI unit of pressure, the pascal (Pa), is defined as 1 newton permetre-squared:
1 Pa =1 N m−2 [1.2a]
In terms of base units, 1 Pa =1 kg m−1s−2 [1.2b]
Several other units are still widely used (Table 1.1); of these units, the most commonly used are atmosphere (1 atm =1.013 25 ×105Pa exactly) and bar (1 bar =105Pa). A pressure of 1 bar is the standard pressurefor reporting data; we denote it p.

If two gases are in separate containers that share a common movable wall (Fig. 1.1), the gas that has the higher pressure will tend to compress (reduce the volume of) the gas that has lower pressure. The pressure of the high-pressure gas will fall as it expands and that of the low-pressure gas will rise as it is compressed. There will come a stage when the two pressures are equal and the wall has no further tendency to move. This condition of equality of pressure on either side of a movable wall (a ‘piston’) is a state of mechanical equilibrium between the two gases. The pressure of a gas is therefore an indication of whether a container that contains the gas will be in mechanical equilibrium with another gas with which it shares a movable wall.
Comment 1.1 The International System of units (SI, from the French Système International d’Unités) is discussed in Appendix1.
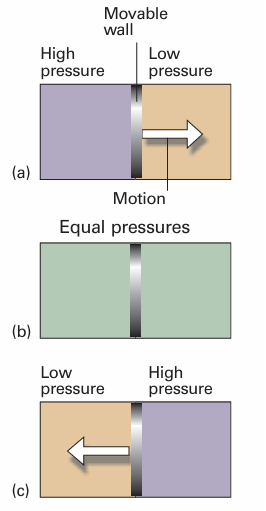
Fig. 1.1When a region of high pressure is separated from a region of low pressure by a movable wall, the wall will be pushed into one region or the other, as in (a) and (c). However, if the two pressures are identical, the wall will not move (b). The latter condition is one of mechanical equilibrium between the two regions.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


