
Critical solution temperatures
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص 186-187
الجزء والصفحة:
ص 186-187
 2025-11-16
2025-11-16
 16
16
Critical solution temperatures
The upper critical solution temperature, Tuc, is the highest temperature at which phase separation occurs. Above the upper critical temperature the two components are fully miscible. This temperature exists because the greater thermal motion over comes any potential energy advantage in molecules of one type being close together. One example is the nitrobenzene/hexane system shown in Fig. 6.19. An example of a solid solution is the palladium/hydrogen system, which shows two phases, one a solid solution of hydrogen in palladium and the other a palladium hydride, up to 300°C but forms a single phase at higher temperatures (Fig. 6.21). The thermodynamic interpretation of the upper critical solution temperature focuses on the Gibbs energy of mixing and its variation with temperature. We saw in Section 5.4 that a simple model of a real solution results in a Gibbs energy of mixing that behaves as shown in Fig. 5.20. Provided the parameter β that was introduced in eqn 5.30 is greater than 2, the Gibbs energy of mixing has a double minimum (Fig. 6.22). As a result, for β > 2 we can expect phase separation to occur. The same model shows that the compositions corresponding to the minima are obtained by looking for the conditions at which ∂∆mixG/∂x = 0, and a simple manipulation of eqn 5.31 shows that we have to solve

The solutions are plotted in Fig. 6.23. We see that, as β decreases, which can be interpreted as an increase in temperature provided the intermolecular forces remain constant, then the two minima move together and merge when β = 2. Some systems show a lower critical solution temperature, Tlc, below which they mix in all proportions and above which they form two phases. An example is water and triethylamine (Fig. 6.24). In this case, at low temperatures the two components are more miscible because they form a weak complex; at higher temperatures the complexes break up and the two components are less miscible. Some systems have both upper and lower critical solution temperatures. They occur because, after the weak complexes have been disrupted, leading to partial miscibility, the thermal motion at higher temperatures homogenizes the mixture again, just as in the case of ordinary partially miscible liquids. The most famous example is nicotine and water, which are partially miscible between 61°C and 210°C (Fig. 6.25).

Fig. 6.22The temperature variation of the Gibbs energy of mixing of a system that is partially miscible at low temperatures. A system of composition in the region P=2 forms two phases with compositions corresponding to the two local minima of the curve. This illustration is a duplicate of Fig. 5.20.
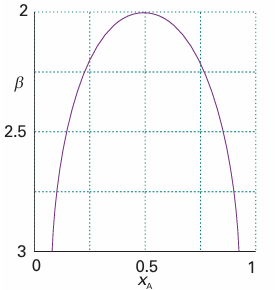
Fig. 6.23The location of the phase boundary as computed on the basis of theβ-parameter model introduced in Section 5.4.
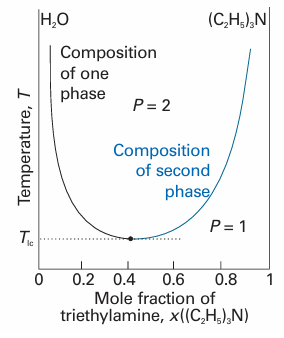
Fig. 6.24The temperature–composition diagram for water and triethylamine. This system shows a lower critical temperature at 292 K. The labels indicate the interpretation of the boundaries.
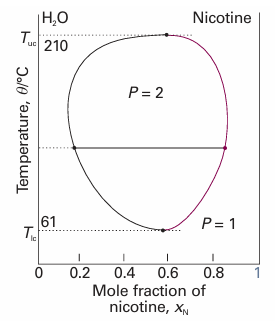
Fig. 6.25The temperature–composition diagram for water and nicotine, which has both upper and lower critical temperatures. Note the high temperatures for the liquid (especially the water): the diagram corresponds to a sample under pressure.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


