
Phase separation
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص185-186
الجزء والصفحة:
ص185-186
 2025-11-16
2025-11-16
 16
16
Phase separation
Suppose a small amount of a liquid B is added to a sample of another liquid A at a temperature T′. It dissolves completely, and the binary system remains a single phase. As more B is added, a stage comes at which no more dissolves. The sample now consists of two phases in equilibrium with each other (P = 2), the most abundant one consisting of A saturated with B, the minor one a trace of B saturated with A. In the temperature–composition diagram drawn in Fig. 6.19, the composition of the former is represented by the point a′ and that of the latter by the point a″. The relative abundances of the two phases are given by the lever rule. When more B is added, A dissolves in it slightly. The compositions of the two phases in equilibrium remain a′ and a″ because P = 2 implies that F′=0, and hence that the compositions of the phases are invariant at a fixed temperature and pressure. However, the amount of one phase increases at the expense of the other. A stage is reached when so much B is present that it can dissolve all the A, and the system reverts to a single phase. The addition of more B now simply dilutes the solution, and from then on it remains a single phase. The composition of the two phases at equilibrium varies with the temperature. For hexane and nitrobenzene, raising the temperature increases their miscibility. The two-phase system therefore becomes less extensive, because each phase in equilibrium is richer in its minor component: the A-rich phase is richer in B and the B-rich phase is richer in A. We can construct the entire phase diagram by repeating the observations at different temperatures and drawing the envelope of the two-phase region.

Fig. 6.19 The temperature–composition diagram for hexane and nitrobenzene at 1 atm. The region below the curve corresponds to the compositions and temperatures at which the liquids are partially miscible. The upper critical temperature, Tuc, is the temperature above which the two liquids are miscible in all proportions.

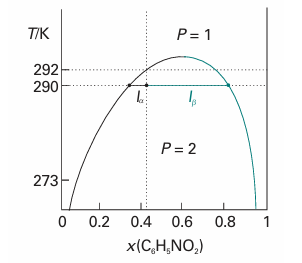
Fig. 6.20 The temperature–composition diagram for hexane and nitrobenzene at 1 atm again, with the points and lengths discussed in the text.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


