


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Presence/absence method BAM/FDA 2011 for Listeria monocytogenes in foods |
|
|
|
Read More
Date: 7-3-2016
Date: 13-3-2016
Date: 21-3-2016
|
Presence/absence method BAM/FDA 2011 for Listeria monocytogenes in foods
Method of the US Food and Drug Administration (FDA), as described in the Bacteriological Analytical Manual (BAM) Online (Hitchins, 2011). Application: all foods.
1. Material required for analysis
Isolation
• Buffered Listeria Enrichment Broth (BLEB)
• Oxford Agar (OXA) plates or Palcam Listeria Selective Agar or Modified Oxford (MOX) Agar or Lithium Chloride Phenylethanol Moxalactam (LPM) Agar with added esculin and Fe3+
• Laboratory incubator set to 30°C
• Laboratory incubator set to 35°C
Confirmation by conventional biochemical tests
• Trypticase Soy Agar with 0.6% Yeast Extract (TSA-YE)
• Trypticase Soy Broth with 0.6% Yeast Extract (TSB-YE)
• Sulfide Indole Motility Medium (SIM) or Motility
Test Medium tubes
• 3% Hydrogen Peroxide (for catalase test)
• Gram Stain Reagents
• Sheep Blood Agar plates
• Purple Broth with 0.5% Dextrose tubes
• Purple Broth with 0.5% Maltose tubes
• Purple Broth with 0.5% Rhamnose tubes
• Purple Broth with 0.5% Xylose tubes
• Purple Broth with 0.5% Mannitol tubes
• Purple Broth with 0.5% Esculin tubes
• Nitrate Broth
• Nitrate Test Reagents (sulfanilic acid solution, α-naphthol solution)
• β-hemolytic Staphylococcus aureus culture (ATCC 49444, ATCC 25923, NCTC 7428 or CIP 5710) (vide note 18.2.2.c.8.1 for changes in nomenclature)
• Rhodococcus equii culture (ATCC 6939 or NCTC 1621)
• Laboratory incubator set to 30°C
• Laboratory incubator set to 35°C
Alternative confirmation using commercial biochemical identification kit
• Vitek Automicrobic Gram Positive and Gram Negative Identification cards (bioMérieux) or API Listeria (bioMérieux) or MICRO-IDTM (bioMérieux) or Phenotype MicroArray for Listeria (Biolog).
2 .Procedure
A general flowchart for detection of Listeria monocytogenes in foods using the presence/absence method BAM/FDA 2011 is shown in Figure1.
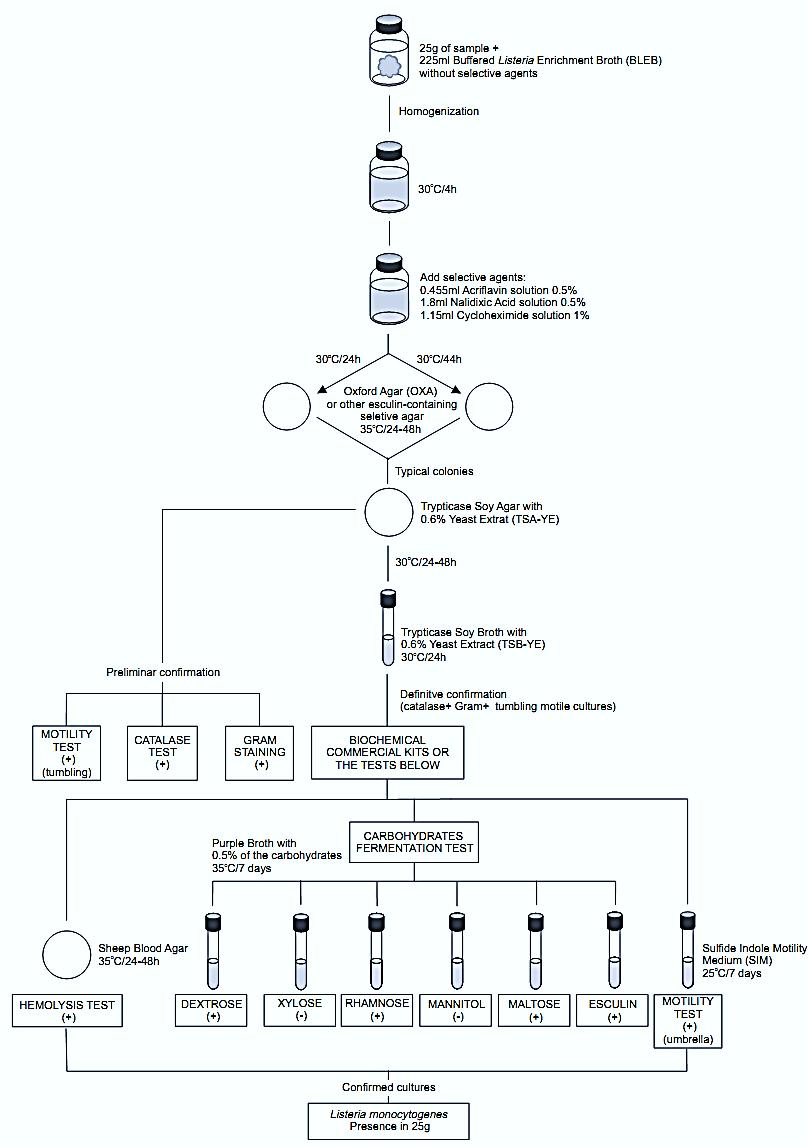
Figure.1 Scheme of analysis for detection of Listeria monocytogenes in foods using the presence/absence method BAM/FDA 2011 (Hitchins, 2011).
The procedure described below does not present these details, as they are supposed to be known to the analyst.
Safety precautions: Pregnant women and immunocompromised persons should avoid handling this microorganism.
a) Pre-enrichment and selective enrichment:
homogenize 25 g or 25 ml of the sample with 225 ml of Buffered Listeria Enrichment Broth (BLEB) containing sodium pyruvate and without the selective agents (acriflavin HCl, nalidixic acid, and cycloheximide). Incubate at 30°C/4 h (pre-enrichment) then add the selective agents and continue incubating at 30°C for a total time of 48 h (selective enrichment).
Note a.1) The method does not specify the acceptable variation for the incubation temperature.
Note a.2) If cycloheximide is unavailable pimaricin (natamycin) may be used at 25 mg/l. If the sample is low in yeast and mold these antifungal agents are not required.
b) Selective-differential plating: From the BLEB culture obtained at 24 and 48 h streak a loopful onto plates of one of the following selective agars containing esculin. Incubate the plates as described for each medium. Listeria colonies are black with a black halo (esculin hydrolysis) on these media.
• Oxford Agar (OXA) incubated at 35°C/24–48 h
• Palcam Listeria Selective Agar incubated at 35°C/24–48 h
• Modified Oxford (MOX) Agar incubated at 35°C/24–48 h
• Lithium Chloride Phenylethanol Moxalactam (LPM) Agar supplemented with esculin and Fe3+ incubated at 30°C/24–48 h
Note b.1) BAM/FDA (Hitchins, 2011) recommends one of the following chromogenic agar in parallel: Biosynth Chromogenic Medium (BCM), Agar Listeria Ottaviani & Agosti (ALOA), Rapid L. mono Medium (RAPID’Lmono), CHROMagar Listeria. Follow manufacturers’ instructions to incubate the plates and read the results.
c) Confirmation: From each selective agar plate select five (or more) typical colonies for confirmation. Purify the culture by streaking onto Trypticase Soy Agar with 0.6% Yeast Extract (TSA-YE). Incubate the TSA-YE plates at 30°C/24–48 h.
Confirm the cultures as L. monocytogenes using the classical tests described below. Alternatively, use a rapid biochemical identification kit as Vitek Automicrobic Gram Positive and Gram Negative Identification cards (bioMérieux) or API Listeria (bioMérieux) or MICRO-IDTM (bioMérieux) or Phenotype MicroArray for Listeria (Biolog).
Select a well isolated bluish typical colony from the TSA plate and test for catalase (c.1), Gram stain (c.2), and motility by wet mount (c.3). From the same colony inoculate a tube of Trypticase Soy Broth with 0.6% Yeast Extract (TSB-YE) and incubate at 30°C/24 h. Use the TSB-YE culture to inoculate the media for tests below (stored at 4°C this culture may be used as inoculum for several days).
c.1) Catalase test: Emulsify a portion of the growth from the TSA colony in one drop of 3% Hydrogen Peroxide on a glass slide. Immediate bubbling is a positive reaction. All Listeria species are catalase positive.
c.2) Gram stain: From the TSA colony test the culture for Gram stain (as described in chapter 5). All Listeria species are short, Gram-positive rods (coccoid cells Gram variable may appear in older cultures).
c.3) Motility using wet mount: From TSA colony prepare a wet mount (as described in chapter 5) to observe motility microscopically under oil immersion. All Listeria species show rotating or tumbling motility. Alternatively, use a semi solid motility test medium.
c.4) Motility using semi solid medium: From the TSB culture inoculate tubes of Sulfide Indole Motility Medium (SIM) or Motility Test Medium (MTM) by stabbing. Incubate the tubes at 25°C for seven days and observe daily. All Listeria species are motile, giving a typical umbrella-like growth.
c.5) Hemolysis test: Mark a grid of 20–25 sections on the bottom of a Sheep Blood Agar plate. From the TSA colony inoculate one culture per section, by stabbing. Inoculate positive and negative controls (positive = L. ivanovii and L. monocytogenes, negative = L. innocua) in parallel. Incubate the plates at 35°C/24–48 h and examine. L.monocytogenes and L. seeligeri produce a slightly hemolysis halo around the stab. L. innocua does not show hemolysis and L. ivanovii produces a well-defined halo around the stab. Doubtful reactions may be confirmed by the CAMP test (c.8).
Note c.5.1) Hemolysis is more easily determined using the blood agar overlay technique: Prepare the blood agar base (without blood) and pour 10 ml per 100 mm diameter Petri dish. Allow to solidify and overlay with 5–6 ml of the complete blood agar medium.
c.6) Carbohydrates fermentation and esculin hydrolysis tests: From the TSB-YE culture, inoculate tubes of Purple Broth with 0.5% of the carbohydrate to be tested (dextrose, esculin, maltose, rhamnose, mannitol, and xylose) (esculin may be omitted if the esculin hydrolysis was clear on the OXA, PALCAM, MOX or LPM plates). The use of Durham tubes for gas collection is optional. Incubate the tubes at 35°C for seven days. Color change from purple to yellow indicates a positive reaction (acid production). Lack of color change indicates a negative reaction. All Listeria species are negative for gas production, positive for esculin hydrolysis and positive for acid pro-duction from dextrose, esculin, and maltose. L. monocytogenes also ferments rhamnose and are negative for xylose and mannitol.
c.7) Nitrate test (optional): From the TSB-YE culture inoculates a tube of Nitrate Broth and incubate at 35°C for five days. Add Nitrate Test Reagents as follows: 0.2 ml of reagent A (sulfanilic acid solution), followed by 0.2 ml of reagent B (α-naphthol solution). An orange color indicates presence of nitrite, i.e. nitrate has been reduced. If no color develops (nitrite absent), test for residual nitrate by adding a small amount of zinc dust. An orange color indicates a negative reaction (nitrate is present, has not been reduced) and the absence of color indicates a positive reaction (nor nitrate or nitrite present, nitrate has been completely reduced to N2). L. monocytogenes does not reduce nitrate.
c.8) CAMP Test (Christie-Atkins-Munch-Peter-son Test) (optional): Inoculate a single-line streak of Staphylocccus aureus ATCC 49444 or ATCC 25923 and a single-line streak of Rhodococcus equi ATCC 6939 on a plate of fresh Sheep Blood Agar. Inoculate a single-line streak of the culture to be tested between and perpendicular to the S. aureus and R. equi streaks (several cultures can be tested in the same plate). Incubate the plates at 35°C/24–48 h and examine for hemolysis. L. monocytogenes and L. seeligeri show enhanced hemolytic reactions near the S. aureus streak. The other species remain non-hemolytic. The reaction of L. monocytogenes may be better after 24 h of incubation than after 48 h.
Note c.8.1) In the catalogue of ATCC (American Type Culture Collection) the current name of the strain N° 49444 is Staphylococcus pseudintermedius, even so it has been originally deposited as Staphylococcus aureus.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Hitchins, A.D. (2011) Detection and enumeration of Listeria monocytogenes . In: FDA (ed.) Bacteriological Analytical Manual, Chapter 10. [Online] Silver Spring, Food and Drug Administration. Available from: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm [accessed 24th October 2011].

|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
شعبة مدارس الكفيل: مخيَّم بنات العقيدة يعزِّز القيم الدينية وينمِّي مهارات اتخاذ القرار لدى المتطوِّعات
|
|
|