


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 20-10-2015
Date: 29-10-2015
Date: 13-10-2015
|
Cytoskeleton
The cytoskeleton is responsible for cell shape, motility (movement) of the cell as a whole, and motility of organelles within a cell. There are three types of filaments in the cytoplasm of most vertebrate cells: microfilaments, microtubules, and intermediate filaments. All of these filament systems share a critical feature: They are composed of proteins that have the unique property of being able to self-assemble into a filamentous network. Imagine a pile of bricks that could assemble by themselves into a wall; the proteins that make up the fibers of the cytoskeleton are able to do just this. The proteins that make each of the three different filament systems assemble into only the structure characteristic of that filament.
Unlike the human skeleton, the cytoskeleton is extremely dynamic, meaning the filament systems are able to lengthen or shorten very rapidly. This dynamic nature of the cytoskeleton is necessary for cells to be able to change shape, complete cell division, or migrate, and represents one of the cytoskeleton’s most important features. Each of the self-assembling proteins has a characteristic concentration, called the “critical concentration,” below which the monomer state is favored and above which the polymer state is favored. Increasingly, the subunit concentration favors filament building, and decreasing it favors filament deconstruction. This property allows the cell to rapidly control cytoskeleton structure.
Microfilaments
The microfilament system is a network of filaments 6 nanometers (nm) in diameter that are important for anchoring plasma membrane proteins, for producing cell movement, and for cell division. The base filament is composed of a protein called actin that is 42 kilodaltons (kd) in weight. Actin is also the protein that forms the thin filaments found in muscle. When purified actin is incubated in a test tube, 6 nm filamentous structures are formed. These threads consist of side-by-side actin monomers that twist around each other in a helix. Inside cells, actin exists in two states, the monomeric protein, called G-actin (for globular actin) and the 6 nm filament, called F-actin (for filamentous actin). The factor that determines the relative proportions of F-actin and G-actin is the concentration of actin protein. Each microfilament has a fast-growing, or “plus,” end, and a slow-growing, or “minus,” end. In most cells the plus ends of the filaments are oriented toward the edge of the cell. In this way rapid polymerization of actin monomers onto the plus ends of microfilaments can produce protrusions on the cell surface called pseudopods. These extensions are critical for the ability of cells to migrate in a directional fashion.
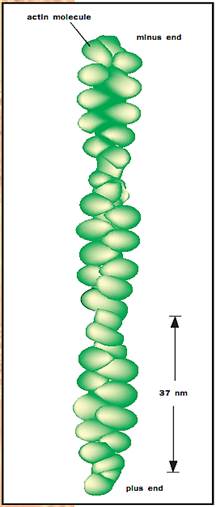
Helical structure of actin molecules.
Microfilaments exist in their highest concentration in association with the cell periphery, where they are believed to play an important role in anchoring membrane proteins. Microfilaments can also be organized into bundles, called stress fibers, which serve as contractile elements, somewhat like little muscles, within cells. These structures are important for maintaining connections between the cell and the surface on which it grows. In addition, these structures may be important for producing contractility to generate directional force during cell motility. A third microfilament-based structure, the contractile ring, is critical for the separation of a cell into its two progeny during cytokinesis.
In most cells the concentration of actin exceeds the critical concentration for microfilament assembly, yet the actin is not entirely assembled into filaments. This occurs because cells make a variety of “actin-associated” or “actin-binding” proteins. One example of an actin binding protein is the G- actin-binding protein profilin. When bound to profilin, actin monomers cannot assemble into filaments. Binding of actin by profilin can effectively reduce the concentration of free actin monomer to below the critical concentration. The actin-binding activity of profilin is regulated in cells. Certain stimuli will cause profilin molecules to release their bound actin monomers, effectively increasing the concentration of actin and thereby stimulating actin assembly. Thus cells can control the relative proportions of G-actin and F-actin.
In general, the functions of actin-associated proteins are to modify the properties of the microfilament network in cells. Some filament-associated proteins, for example the protein tropomyosin, bind along the length of the filament to stiffen it. There are also proteins such as villin or filamin that bind microfilaments together side by side to produce bundles of actin filaments. Other actin-binding proteins cross-link actin filaments to form meshlike structures such as those found in association with the cell membrane. Cells can also control the length of filaments through the action of proteins that can cut filaments to produce two shorter filaments. To keep the filaments a certain length, cells produce “capping” proteins that bind to the ends and prevent the addition of new actin subunits. By modulating the state of the microfilament network the cell can control the physical properties of the cytoplasm such as rigidity and viscosity.
One of the most interesting types of actin-associated proteins is a family of enzymes, called myosins, which have the ability to convert chemical energy into movement. The characteristic property of these so-called myosin molecular motors is their ability to bind actin in an adenosine triphosphate-sensitive fashion and to produce movement of actin filaments. Over fifteen different types of myosin motors have been identified. Some of them, such as those involved in cytokinesis and cell motility, are two headed, meaning they have two actin-binding motor domains, while others have only one head. Some of these myosins are involved in the movement of membrane-bound vesicles along actin tracks. The best characterized of these molecular motors, myosin II, slides actin filaments past each other either to power contraction of the contractile ring or to produce cell migration. A different version of this myosin motor forms the thick filaments that are responsible for the contraction of muscle.
Microtubules
Microtubules are the largest of the cytoskeletal filaments with a diameter of 25 nm. There are many parallels between the microfilament cytoskeletal system and the microtubule system. Like microfilaments, microtubules are produced by the self-assembly of a subunit, which in the case of microtubules is a heterodimer composed of one alpha tubulin and one beta tubulin bound together. Alpha and beta subunits alternate to form a protofilament. Thirteen protofilaments line up side by side, forming the hollow tube of the microtubule.
Microtubules also have a fast-growing, or plus, end and a slow-growing, or minus, end. In most cells microtubules are organized in a radial array extending from a single site termed the microtubule organizing center (MTOC), generally positioned near the nucleus. This organization produces a network of microtubule tracks where the plus ends of the microtubules are near the cell surface and the minus ends are associated with the MTOC. This structure is well suited for the primary function of microtubules, which is to serve as tracks along which membrane-bound vesicles are moved. Vesicles transported include organelles such as mitochondria, as well as secretory vesicles destined for exocytosis.
Another parallel with microfilaments is the highly dynamic nature of microtubules. Microtubules exhibit a phenomenon called “dynamic instability.” Individual microtubules constantly grow and shorten, often shortening dramatically in a process called “catastrophe.” This rapid turnover of microtubules allows cells to change shape quickly and facilitates reorganization of the tracks important for delivery of vesicles to sites throughout the cell. Like the microfilament cytoskeleton, the dynamics of microtubules can be modified by microtubule associated proteins, called MAPs. Some MAPs stabilize microtubules, while others cross-link microtubules, both with other microtubules as well as with microfilaments and the third cytoskeletal system, intermediate filaments (see below).
The dynamics of microtubules are also important for mitosis. Each time the cell goes through division the microtubule network is completely disassembled and the tubulin subunits are reassembled into a new structure called the spindle. The spindle is responsible for the segregation of chromosomes into each daughter cell and also plays an important role in specifying the position of the cleavage plane that will separate the two daughter cells (during cytokinesis).
The functions of microtubules in vesicle transport and chromosome segregation are dependent on molecular motors that bind to and move along microtubule tracks. These motors are divided into two families, kinesin and cytoplasmic dynein. Kinesin was the first microtubule motor to be identified. It is responsible for moving vesicles (the cargo of the motor) toward the plus ends of microtubules, that is, from the center of the cell toward the plasma membrane. Since discovery of the first kinesin, the family has been shown to consist of many members, some of which are important for spindle function during mitosis. Some of these kinesins move toward the minus ends of microtubules. In contrast, the other type of microtubule motor, cytoplasmic dynein appears to move cargo exclusively toward the minus ends of microtubules, that is, from the cell periphery back towards the center.
The ability of these motors to move organelles around inside of cells is critical for processes such as hormone secretion, transmission of nerve impulses and recycling of membrane.
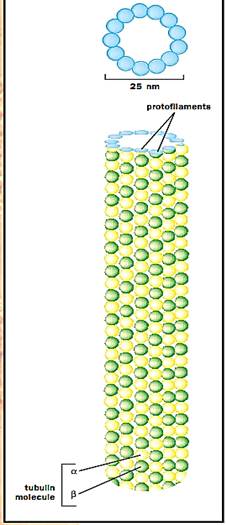
Top: Illustration of the ring of 13 distinct subunits in a microtubule, each of which corresponds to a tubulin molecule. Bottom: A side view of a section of a microtubule, with the tubulin molecules in long, parallel rows called protofilaments.
Intermediate Filaments
The third cytoskeletal system is called the intermediate filament system because the filaments, which are 10 nm in diameter, are intermediate in size between microfilaments and microtubules. There are many other features that set the intermediate filaments system apart from the other cytoskeletal systems. Unlike the other systems, which are composed of one or two different proteins, intermediate filaments can be formed by a relatively large number of different proteins. For example, the primary intermediate filaments found in epithelial cells (such as skin) are formed from pairs of keratins, one basic and one acidic. There are a large number of different keratin pairs, found in different tissues, that produce 10-nm filaments. Wool, hair, and nails are examples of structures formed from intermediate filaments. The different filament-forming keratins are developmentally regulated, and the keratins expressed early in embryos differ from those expressed later in development.
In contrast, a different cell type, fibroblasts, have intermediate filaments that are formed from a single protein, vimentin. In heart tissue, the intermediate filaments can be formed from a different single protein, desmin. In nervous tissue the intermediate filaments are formed from yet another family of intermediate filament proteins called neurofilament proteins. There are even structures in the nucleus formed from intermediate filament protein family members called nuclear lamins.
Although intermediate filaments can also self-assemble from their constituent subunits, the filaments differ from microtubules and microfilaments in that they do not have an obvious polarity. Structurally, intermediate filaments are formed from a bundle of subunit proteins which themselves are extended in structure, as compared to the more globular-shaped protein subunits that form microfilaments and microtubules. Intermediate filaments are generally more stable structures than the other cytoskeletal systems, although recently it has been shown that subunits are capable of exchanging in and out of the filament all across their length. Like other filament systems, intermediate filaments have associated proteins, but interestingly no molecular motors that use intermediate filaments as their track have been identified.
Intermediate filaments are organized within cells so that they link the cell surface and the nucleus. Intermediate filaments are believed to play an important role in cells by stabilizing structural integrity. Of all the cy- to skeletal systems, intermediate filaments are best suited to play this structural role since they have the highest tensile strength (resistance to stretch). At the cell surface, intermediate filaments attach to specific junctions called desmosomes and hemidesmosomes. These junctions attach cells to neighboring cells or the extracellular matrix.
Mutations in intermediate filament subunit proteins have been shown to cause human diseases. For example, mutations in keratins cause blistering diseases that result from a loss of cellular integrity, causing cells to lit -erally split in half. Similarly, mutations in the neurofilament proteins produce neurological diseases called neuropathies.
Cytoskeleton-Based Cellular Structures
Several cellular structures are built around a core of cytoskeletal proteins. Perhaps the best known examples are cilia and flagella. Flagella provide the motive force for sperm motility through their waving motion. Cilia line the surfaces of cells in the respiratory tract where their motion constantly moves mucus along the airway surface. The core of both flagella and cilia is composed of a highly organized bundle of specialized microtubules. Around a “central pair” of microtubules, there are nine pairs of modified microtubules called “doublet microtubules.” The central pair and the outer doublet microtubules are connected by a number of different specialized proteins. The characteristic waving motion of cilia and flagella is generated by the action of a microtubule-based motor called axonemal dynein that moves the microtubules in the flagellum relative to each other. Axonemal dynein is related to the minus end directed motor cytoplasmic dynein that moves vesicles along microtubules. Dynein mutation causes cilia dysfunction, leading to respiratory illness and sperm immotility. Curiously, about half of the people with these mutations also have “situs inversus,” in which the internal organs are reversed left for right.
Another microtubule-based cellular structure is the centriole. The cen- triole is a somewhat mysterious cylindrical structure containing vanes formed from microtubules that run the length of the cylinder. Centrioles together with the associated pericentriolar material form a somewhat larger structure called a centrosome. Centrosomes function as microtubule organizing centers during interphase of the cell cycle, and become the center of the spindle poles during mitosis.
Finally, several cell types such as intestinal epithelial cells have protrusions from their surface called microvilli. At the core of the mirovilli are bundles of actin filaments. These protrusions are believed to increase the surface area of the intestinal cells to maximize their ability to absorb nutrients.
References
Alberts, Bruce et al. The Molecular Biology of the Cell, 4th ed. New York: Garland Publishing, 2000.
Bray, Dennis. Cell Movements. New York: Garland Press, 1992.
Lodish, Harvey, et al. Molecular Cell Biology, 3rd ed. New York: Scientific American Books, 1995.

|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
العتبة العباسية المقدسة تجري القرعة الخاصة بأداء مناسك الحج لمنتسبيها
|
|
|