


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 29-5-2017
التاريخ: 19-9-2019
التاريخ: 2025-01-11
التاريخ: 28-5-2017
|
Another feature of E1 reactions (and also of SN1 reactions) is the tendency of the initially formed carbocation to rearrange, especially if a more stable carbocation is formed thereby. For example, the very slow SN1 solvolysis of neopentyl iodide in methanoic acid leads predominantly to 2-methyl-2-butene:
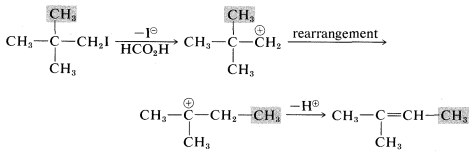
In this reaction, ionization results in migration of a methyl group with its bonding pair of electrons from the β to the α carbon, thereby transforming an unstable primary carbocation to a relatively stable tertiary carbocation. Elimination of a proton completes the reaction.
Rearrangements involving shifts of hydrogen (as H:⊖) occur with comparable ease if a more stable carbocation can be formed thereby:
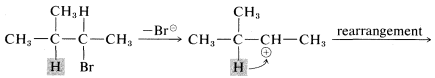
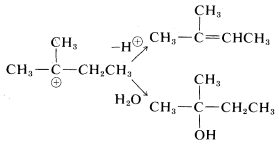
Rearrangements of carbocations are among the fastest organic reactions known and must be reckoned with as a possibility whenever carbocation intermediates are involved.



|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
العتبة العباسية المقدسة تجري القرعة الخاصة بأداء مناسك الحج لمنتسبيها
|
|
|