


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-3-2016
Date: 22-10-2020
Date: 19-10-2020
|
Alkyl side chains are activated towards oxidation at the benzylic position, because free radicals are stabilized at that position through resonance. When heated with aqueous potassium permanganate (KMnO4) under acidic conditions, alkylbenzenes are oxidized to benzoic acids, as long as the alkyl group contains at least one hydrogen at the benzylic position. This means that tert-butylbenzene (shown below) does not react. Aryl methyl ketones (acetophenones), made by the Friedel-Crafts reaction, can also be oxidized to benzoic acids using hot aqueous permanganate.

The benzylic C-H bonds weaker than most sp3 hybridized C-H.

Because of the weak C-H bonds, benzylic hydrogens can form benzylic halides under radical conditions.

NBS (N-bromosuccinimide) is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts with trace amounts of HBr to produce a low enough concentration of bromine to facilitate the benzylic bromination reaction via a radical process.
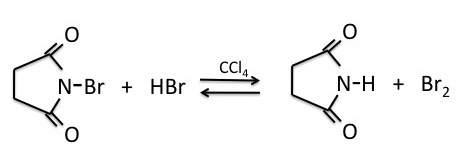



|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تجري القرعة الخاصة بأداء مناسك الحج لمنتسبيها
|
|
|