
Copper electron-transfer centres
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
745
الجزء والصفحة:
745
 2025-10-23
2025-10-23
 56
56
Copper electron-transfer centres
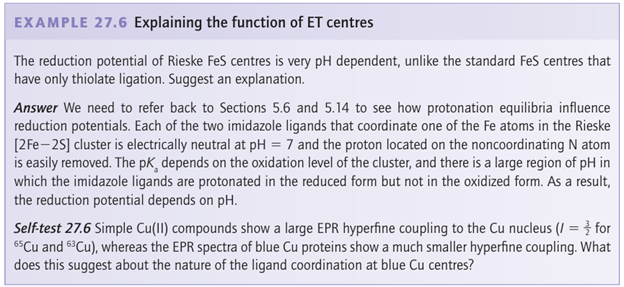
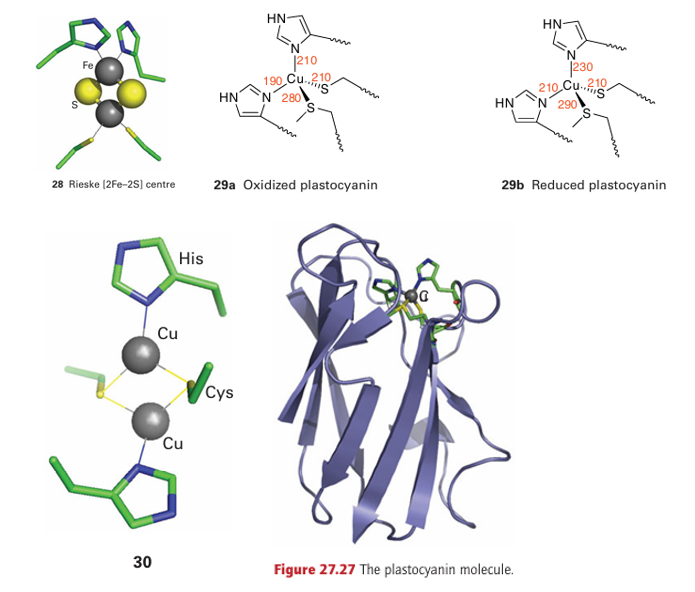
Key point: The protein overcomes the large inherent difference in preferred geometries for Cu (II) and Cu(I) by constraining Cu in a coordination environment that does not change upon electron transfer. The so-called ‘blue’ Cu centre is the active site of a number of small electron-transfer prteins as well as larger enzymes (the blue Cu oxidases) that contain, in addition, other Cu sites. Blue Cu centres have reduction potentials for the Cu (II)/Cu(I) redox couple that lie in the range 0.15 0.8 V and so they are generally more oxidizing than cytochromes. The name stems from the intense blue colour of pure samples in the oxidized state, which arises from ligand(thiolate)-to-metal charge transfer. In all cases, the Cu is shielded from solvent water and coordinated by a minimum of two imidazole-N and one cysteine-S in a nearly trigonal planar manner, with one or two longer bonds to axial ligands. The most studied examples are plastocyanin (Fig. 27.27), a small electron carrier protein in chloroplasts, and azurin, a bacterial electron carrier. These small proteins have a β-barrel structure, which holds the Cu coordination sphere in a rigid geometry. Indeed, the crystal structures of oxidized, reduced (29, numbers refer to bond distances in pm), and apo forms reveal that the ligands remain in essentially the same position in all cases. As a result, the blue Cu centre is well suited to undergo fast and efficient electron transfer because the reorganization energy is small. The dinuclear Cu centre known as CuA is present in cytochrome c oxidase and N2O re ductase. The two Cu atoms (30) are each coordinated by two imidazole groups and a pair of cysteine thiolate ligands act as bridging ligands. In the reduced form, both Cu atoms are Cu(I). This form undergoes one-electron oxidation to give a purple, paramagnetic species in which the unpaired electron is shared between the two Cu atoms. Once again, we see how delocalization assists electron transfer because the reorganization energy is lowered.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


