
Cytochromes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص742-743
الجزء والصفحة:
ص742-743
 2025-10-23
2025-10-23
 47
47
Cytochromes
Key points: Cytochromes operate in the potential region 0.3 to 0.4 V; they have a combination of low reorganization energy and extended electron coupling through delocalized orbitals. Cytochromes were identified many years ago as cell pigments (hence the name). They contain an Fe porphyrin group and the term ‘cytochrome’ can refer to both an individual protein and a subunit of a larger enzyme that contains the cofactor. Cytochromes use the Fe3 /Fe2 couple and are generally six-coordinate (23), with two stable axial bonds to amino acid donors, and the Fe is usually low spin in both oxidation states. This contrasts with ligand-binding Fe-porphyrin proteins such as haemoglobin, for which the sixth coordination site is either empty or occupied by an H2O molecule. A good way to consider the capability of cytochromes for fast electron transfer is to treat the d orbitals of Fe (III) and Fe(II) in terms of an octahedral ligand field and to consider the overlap between the electron-rich but nearly nonbonding t2g orbitals (the configurations are t2g5 and t2g 6 in Fe(III) and Fe(II), respectively) and the orbitals of the porphyrin. The electron enters or leaves an orbital having π overlap with the π* antibonding molecular orbital on the ring system. This arrangement provides enhanced electron transfer because the d orbitals of the Fe atom are effectively extended out to the edge of the porphyrin ring, so decreasing the distance over which an electron must transfer between redox partners (Fig. 27.23). The paradigm of cytochromes is mitochondrial cytochrome c (12 kg mol 1, Fig. 27.24). This cytochrome is found in the mitochondrial intramembrane space, where it supplies electrons to cytochrome c oxidase, the enzyme responsible for reducing O2 to H2O at the end of the energy-transducing respiratory chain (Section 27.10). The fifth and sixthlig ands to Fe in cytochrome c are histidine (imidazole-N) and methionine (thioether-S, 23). Methionine is not a common ligand in metalloproteins but because it is a neutral, soft
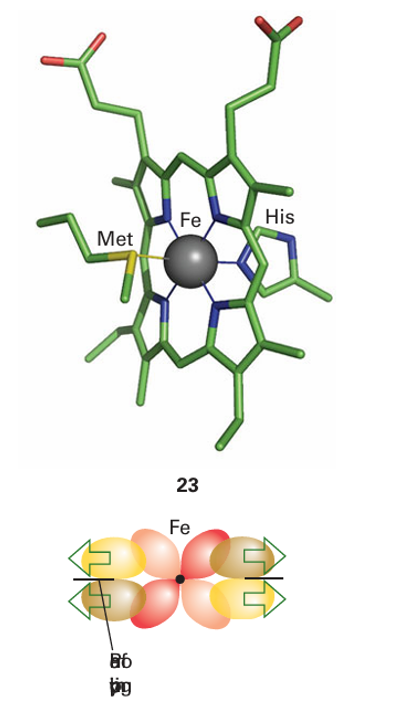
Figure 27.23 Overlap between the t2g orbitals of the Fe and low-lying empty π* orbitals on the porphyrin effectively extends the Fe orbitals out to the periphery of the ring.
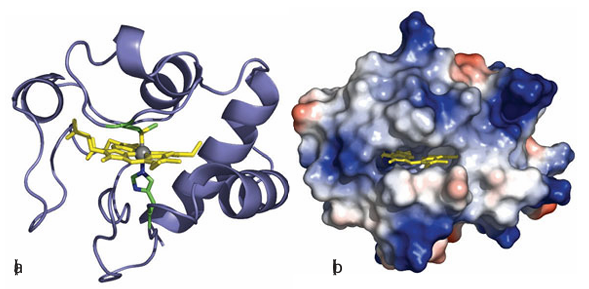
Figure 27.24 Different views (but from the same viewpoint) of mitochondrial cytochrome c. (a) The secondary structure and the position of the haem cofactor. (b) The surface charge distribution that guides the docking with its natural redox partners (red and blue areas represent patches of negative and positive charge, respectively). donor it is expected to stabilize Fe (II) rather than Fe(III). The reduction potential of cyto chrome c is 0.26 V, at the higher end of values for cytochromes in general. Cytochromes vary in the identity of the axial ligands as well as the structure of the porphyrin ligand (the notation a, b, c, d… defines positions of absorption maxima in the visible region, but also refers to variations in the substituents on the porphyrin ring). Many cytochromes, in particular those sandwiched between membrane-spanning helices, have bis(histidine) axial ligation (24). In cytochrome c, the edge of the porphyrin ring is exposed to solvent and is the most likely site for electrons to enter or leave. Specific protein–protein interactions are import ant for obtaining efficient electron transfer and the region around the exposed edge of the porphyrin ring in cytochrome c provides a pattern of charges that are recognized by cytochrome c oxidase and other redox partners. One example in particular has been well studied, that of cytochrome c with yeast cytochrome c peroxidase. The driving force for electron transfer from either of the two catalytic intermediates of peroxidase (Section 27.10) to each reduced cytochrome c is approximately 0.5 V. Figure 27.25 shows the structure of a bimolecular complex formed between cytochrome and cytochrome c peroxidase. Electrostatic interactions guide the two proteins together within a distance that is favourable for fast electron tunnelling between cytochrome c and two redox centres on the peroxidase, the haem cofactor and tryptophan-191 (Section 27.10).
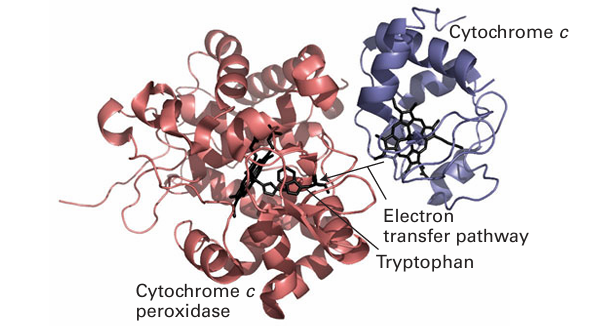
Figure 27.25 The bimolecular ET complex between cytochrome c and cytochrome c peroxidase produced by co-crystallization of cytochrome c with the Zn derivative of cytochrome c peroxidase. The orientation suggests an electron transfer pathway between the haem groups of cytochrome c and cytochrome c peroxidase that includes tryptophan.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


