


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 3-3-2016
Date: 13-3-2016
Date: 2025-01-01
|
Vibrio cholerae and Vibrio parahaemolyticus
1. Introduction
The members of the genus Vibrio are primarily aquatic bacteria, found both in freshwater and in seawater, in addition to being frequently associated with marine animals. Several species cause diarrhea or infections of the gastrointestinal tract but the most frequent enteric pathogens are Vibrio cholerae and Vibrio parahaemolyticus (Farmer III et al., 2005). Sporadic illnesses have been attributed to Vibrio vulnificus, but no major outbreaks have been reported (Jones, 2012c).
Cholera, a disease caused by strains of V. cholerae of serotypes O1 and O139, is classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) into risk group IA: “diseases of severe hazard for general population; life threatening or resulting in substantial chronic sequelae or presenting effects of long duration”.
The disease caused by V. parahaemolyticus is classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) into risk group III: “diseases of moderate hazard usually not life threatening, normally of short duration without substantial sequelae, causing symptoms that are self-limiting but can cause severe discomfort”.
The diseases caused by V. vulnificus are classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) into risk group IB: “diseases of severe hazard for restricted population; life threatening or resulting in substantial chronic sequelae or presenting effects of long duration”. The at-risk groups are individuals with high iron blood levels or who suffer from liver disorders, related to the high consumption of alcohol.
Other Vibrio species have also been isolated from clinical specimens, indicating that they are pathogenic to humans. The species found in intestinal specimens include V. alginolyticus, V. fluvialis, V. furnissii, V. hollisae and V. mimicus. Species found to occur in extraintestinal specimens include V. cincinnatiensis, V. damsela, V. harveyi and V. metschnikovii (Farmer III et al., 2005).
2. Taxonomy
Vibrio is a genus of the family Vibrionaceae, defined as motile Gram-negative straight-or curved rods, non-spore-forming. They are facultative anaerobes, with both respiratory and fermentative metabolism. The majority of the species is oxidase-positive, reduce nitrate to nitrite and use glucose as sole source of carbon and energy (Farmer III and Janda, 2005).
In the 1st Edition of Bergey’s Manual of Systematic Bacteriology (Baumann and Schubert, 1984), the family Vibrionaceae included the genera Vibrio, Photobacterium, Aeromonas and Plesiomonas. In the 2nd Edition (Farmer III and Janda, 2005) several changes were introduced into the classification, with the family now including the genera Vibrio, Photobacterium, Salinivibrio, Allomonas and Listonella.
The genus Aeromonas was transferred to the family Aeromonadaceae and the genus Plesiomonas to the family Enterobacteriaceae (Farmer III and Janda, 2005). These microorganisms are also aquatic and, eventually, may be isolated along with the vibrios. Plesiomonas occurs in freshwater and marine environments and in habit-ants of those ecosystems (Janda, 2005). Aeromonas is not a marine bacteria, but may be encountered in sea systems that have a freshwater interface, in fresh water, estuarine water, surface water and drinking water, and in association with aquatic animals (Martin-Carnahan and Joseph, 2005).
Allomonas and Listonella are new genera that had not yet been separately described in the 2nd edition of Bergey’s Manual. The species classified into these genera were previously listed as vibrios and continue to be described as such: Allomonas enterica as Vibrio fluvialis, Listonella anguillarum as Vibrio anguillarum and Listonella pelagia as Vibrio pelagius (Farmer III and Janda, 2005).
According to Janda (2005) one of the main characteristics that distinguish the genera of the family Vibrionaceae from each other and from Aeromonas and Plesiomonas are the resistance to 150μg of the vibrio-static agent O/129 (2,4-diamino-6,7-diisopropylpterid-ine phosphate): Vibrio generally sensitive and Aeromonas and Plesiomonas generally resistant. At lower concentrations (10μg) it is also used to differentiate some Vibrio species from each other. The “string test”, which verifies the lysis of the cells in the presence of 0.5% sodium deoxycholate, is also used to differentiate Vibrio (generally positive) from Aeromonas and Plesiomonas (generally negative). The capacity to grow in the absence of NaCl, but not at a concentration of 6% differentiates Aeromonas and Plesiomonas from halophilic vibrios.
Description of the genus Vibrio (from Farmer III et al., 2005): Cells are small, slightly curved, curved or comma-shaped rods, non-spore-forming, Gram negative, motile. Chemoorganotrophs, facultative anaerobes with both respiratory and fermentative metabolism, they ferment glucose producing acid but rarely gas. All species except V. cholerae and V. mimicus have an absolute requirement for Na+, which in some instances may be partially compensated by concentrations of Mg+ or Ca+ similar to those normally found in sea-water. Oxidase positive. Several species grow at 4°C, all grow at 20°C, most grow at 30°C and many grow at 35–37°C. Most species prefer the pH range of 7,0 to 8,0 for growth.
The 2nd edition of Bergey’s Manual (Farmer III et al., 2005) also contains tables describing the principal characteristics differentiating the 12 vibrio species that are pathogenic to man. To facilitate differentiation, these species can be divided into six groups, depicted in Table.1. Group one includes the pathogenic vibrios that are capable of growing in the absolute absence of NaCl (V. cholerae and V. mimicus), Group two includes the oxidase- and nitrate-negative species (V. metschnikovii), Group three includes those that ferment inositol (V. cincinnatiensis), Group four brings together the species that are negative for arginine and lysine (V. hollisae),
Group five includes the species that are arginine-positive (V. damsela, V. fluvialis and V. furnissii), and Group six includes those that are arginine-negative and lysine-positive (V. alginolyticus, V. harveyi, V. parahaemolyticus and V. vulnificus). The remaining characteristics of the pathogenic species are described in Table.2.
V. cholerae is the type-species of the genus Vibrio and one of the most important from an epidemiological standpoint, although not all strains are pathogenic. The species is divided into about 180 somatic serotypes and the strains responsible for the epidemics and pandemics of cholera belong to serotype O1 or serotype O139. The serotype O1 strains are further subdivided into the subtypes Inaba, Ogawa and Hikojima, which present antigenic formulas of the AB, AC and ABC type, respectively (Farmer III et al., 2005).
Table.1 Key characteristics used to differentiate the pathogenic Vibrio in groups a,b (Farmer III et al., 2005).
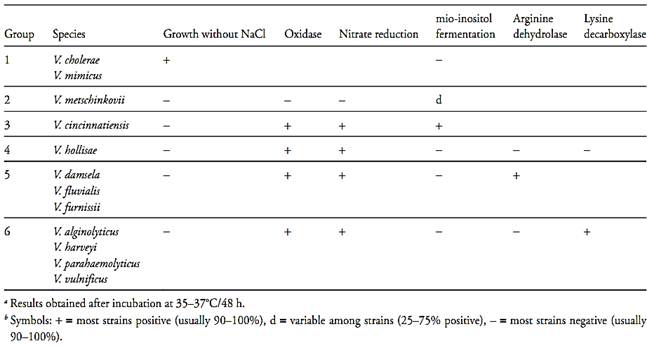
Table.2 Biochemical characteristics of pathogenic Vibrio (Farmer III et al., 2005).
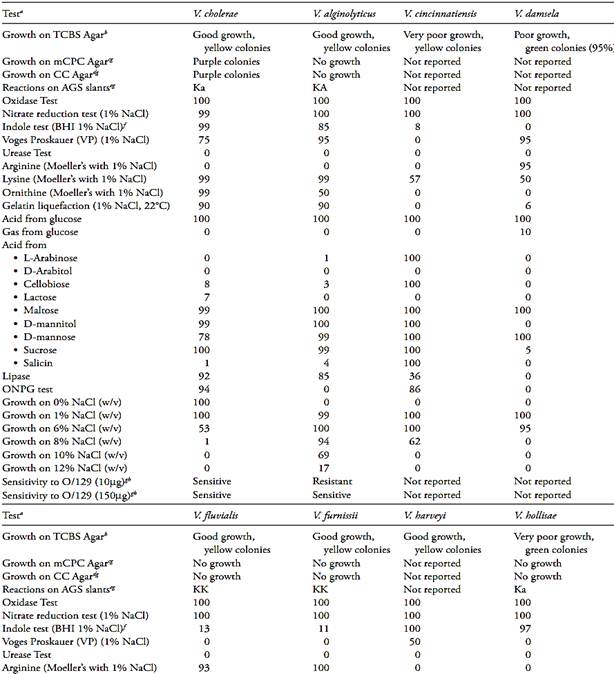
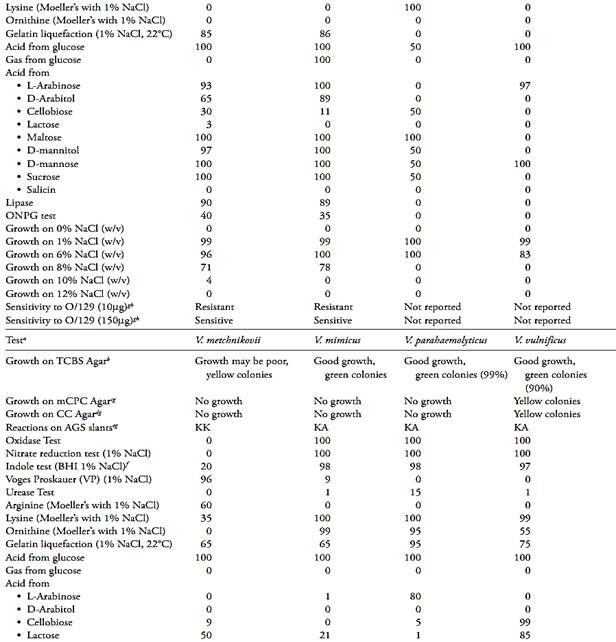
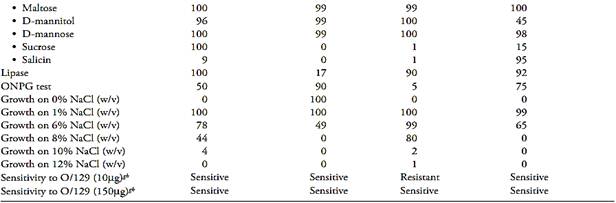
a The results related by Farmer III et al. (2005) is the percentage of strains showing a positive result after 48 h of incubation at 36°C (except when reported other condition). The NaCl concentration of the test media was adjusted to 1% (w/v).
b Growth and colonies color on Thiosulfate Citrate Bile Sucrose (TCBS) Agar.
c Growth and colonies color on Modified Cellobiose Polymyxin Colistin (mCPC) Agar.
d Growth and colonies color on Cellobiose Colistin (CC) Agar.
e Reaction on Arginine Glucose Slants (AGS), where KK = slant and butt alkaline, KA = slant alkaline and butt acidic, Ka = slant alkaline and butt slightly acidic.
f Brain Heart Infusion Broth with 1% NaCl.
g Data from Bacteriological Analytical Manual Online (Kaysner and De Paola, 2004).
h Vibriostatic agent 2,4-diamino-6,7-diisopropylpteridine.
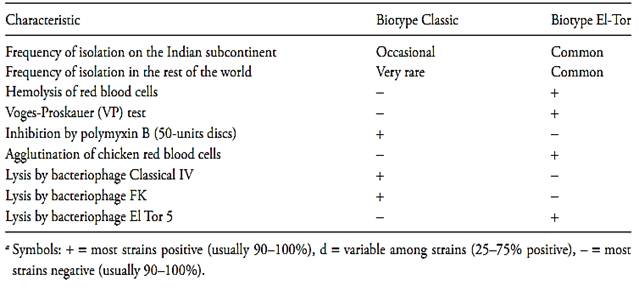
A second subdivision of the 01 strains of V. cholerae, based on their phage-resistance characteristics, their resistance to polymyxin and other biochemical characters, distinguishes two biotypes of V. cholerae: the classical biotype and the El-Tor biotype (Farmer III et al., 2005). The main characteristics distinguishing these two biotypes from each other are described in Table.3.
According to Farmer III et al. (2005) not all strains of V. parahaemolyticus are pathogenic. The pathogenic strains are distinguished from the non-pathogenic by the production of a heat-stable hemolysin known as “Thermostable Direct Hemolysin” (TDH). The production of TDH can be detected through hemolysis of human erythrocytes in Wagatsuma Agar, a reaction known as the Kanagawa phenomenon or reaction.
Virtually all strains that cause gastroenteritis in humans are Kanagawa-positive, whereas those isolated from sea-water or seafood are almost always negative. Kanagawa negative strains associated with gastroenteritis have been found and they produce a different hemolysin named “TDH-related-hemolysin” and abbreviated as “TRH”. The gene for TRH has 70–80% homology to the gene for TDH and appears to be closely associated with urease expression.
3. Epidemiology
Three species of Vibrio are responsible for most cases of human illness caused by vibrios, associated with several seafood vehicles: V. cholerae, V. parahaemolyticus and V. vulnificus.
Table.3 Characteristics used to differentiate V. cholerae O1 biotypes classic and ElTora (Farmer III et al., 2005).
3.1 V. cholerae
According to WHO (2005) V. cholerae O1 and O139 are the causative agents of cholera, a water and food-borne disease with epidemic and pandemic potential. The infection varies from mild to severe gastrointestinal illness; about 20% of patients develop acute, watery diarrhea and 10 to 20 % develop severe watery diarrhea with vomiting. Without treatment dehydration and death can occur within hours and the mortality rate may reach 30–50%. However, with adequate treatment the mortality rate is less that 1%. The infective dose is about 106 cells and, according to Jones (2012a) the incubation time varies from few hours to three days after ingestion. The symptoms are caused by a toxin which acts on the epithelial cells of the intestinal mucosa and causes secretion of electrolytes and water. The mild cases usually have a duration of few days and the cases requiring rehydration therapy or antibiotic treatment can persist longer, depending on the severity of illness when the treatment is initiated.
According to WHO (2005) the primary source of choleragenic V. cholerae is the faeces of persons acutely infected with the organism and thus it reaches water most often through sewage. V. cholerae can survive in water for long periods but appears to be confined to fresh water and estuarine environments. According to Jones (2012a) the infections with these organisms in areas where serogroups O1 and/or O139 are endemic, infections can occur from ingestion of water; ice; unwashed, contaminated food; and seafood. In U.S.A the infections have been associated with molluscan shellfish (oysters, mussels, and clams), crab, lobster, shrimp, squid, and finfish, consumed raw, improperly cooked, or cross contaminated by a raw product.
3.2 V. parahaemolyticus
According to Jones (2012b) the pathogenic strains of V. parahaemolyticus cause gastroenteritis characterized by diarrhea, stomach cramps, fever, nausea, and/or vomiting, and, occasionally, bloody diarrhea.
The disease is caused by the ingestion of foods contaminated with pathogenic strains and the infective dose is generally greater than 106 cells. The incubation period normally varies from 4 to 90 hours after ingestion and duration varies from two to six days. The disease is generally mild or moderate, whit less than 40% of reported cases requiring hospitalization and/or antibiotic treatment. The mortality rate is 2%. In susceptible people (patients with diabetes, liver disease, kidney disease, cancer, AIDS, immunosuppressive medications) septicemia may occur, whit a mortality rate of 20–30%.
According to WHO (2011) Vibrio parahaemolyticus is a marine micro-organism native in estuarine waters throughout the world. The pathogenic strains cause disease worldwide, although most common in Asia and the U.S.A. Molluscan shellfish, such as oysters, clams and mussels raw or undercooked are the most common food associated with V. parahaemolyticus infection.
3.3 V. vulnificus
According to Jones (2012c) the ingestion of this organ-ism can cause gastroenteritis characterized by fever, diarrhea, abdominal cramps, nausea, and vomiting. The symptoms occur within 12 h and 21 days and the dis-ease generally remains localized and is self-limiting. In susceptible people (immunocompromised or with liver disease), V. vulnificus may cause primary septicemia (septic shock) characterized by fever and chills, occasionally accompanied by vomiting, diarrhea, abdominal pain, and/or pain in the extremities. The mortality rate is 35% and in more than 60% of the cases the patients develop secondary lesions on the extremities.
V. vulnificus also can cause wound infections characterized by inflammation at the wound site, which can progress to cellulitis, bullous lesions, and necrosis. The mortality rate is 20% and the infection can become systemic, causing fever, chills, altered mental status, and hypotension.
The marine environment is the natural habitat of Vibrio vulnificus and sporadic cases may occur in the hot months of the year. The mode of transmission is the ingestion of contaminated foods or contamination of wounds. The foods most frequently associated with this pathogen are raw or undercooked oysters, clams and shrimp (Jones, 2012c).
4. Methods of analysis
The culturing methods of the Food and Drug Administration (FDA) (Kaysner and De Paola, 2004), the American Public Health Association (APHA) (Kaysner and De Paola, 2001), and the International Organization for Standardization (ISO 21872-1:2007/Cor.1:2008) for isolation or enumeration of vibrios in foods are based, principally, on the ability of these microorganisms to grow under alkaline conditions and in the presence of relatively high concentrations of bile salts. They generally include an enrichment step, which limits the counts to the most probable number technique.
The enrichment step utilizes Alkaline Peptone Water (APW), with a pH value between 8,4 and 8,6, both for V. cholerae, as for V. parahaemolyticus and V. vulnificus.
In the analysis of V. cholerae, the incubation period should not be very prolonged, to avoid the risk of pre-dominance by competing microbiota may obscure the results. The recommended incubation time is 6–8 h, however, for processed foods, samples should be plated after 6–8 h and again after 18–21 h. Some laboratories opt for overnight incubation (16–18 h), to perform laboratory work during business hours. This practice, however, is less advisable and should be avoided when-ever possible. For the selective differential plating, the most widely used medium is Thiosulfate Citrate Bile Sucrose (TCBS) Agar, especially developed for the isolation of vibrios in general. This growth medium explores the resistance to bile and the capacity to grow under alkaline conditions (pH 8.6) as main selective characteristics.
The differentiation characteristic between the species is the fermentation or not of sucrose, with positive cells producing yellow colonies and negative strains producing green colonies. TCBS Agar is used for isolating V. cholerae (yellow colonies), V. parahaemolyticus (green colonies) and V. vulnificus (green colonies). V. mimicus, which is closely related to V. cholerae, may be differentiated on TCB by the ability to ferment sucrose. Modified Cellobiose Polymyxin Colistin (m-CPC) Agar and Cellobiose Colistin (CC) Agar are recommended for preferential isolation of V. vulnificus, both of which are culture media that utilize the fermentation of cellobiose at 39–40°C as differential characteristic.
Several other vibrios and most strains of V. parahaemolyticus do not grow in these media. As a function of this, they are also recommended to be used as second plating medium in V. cholerae analysis, to reduce interference from other vibrios. The strains of the classical V. cholerae biotype, sensitive to polymyxin, will not grow in m-CPC, but these strains are very rare.
To screen for presumptive strains the characteristics most used are the production of arginine dihydrolase, gas and H2S. V. cholerae, V. parahaemolyticus and V. vulnificus all have the same negative profile for these tests, but are differentiated by verifying their capacity to grow in the absence of NaCl (V. cholerae grows, V. parahaemolyticus and V. vulnificus do not grow).
Verification of motility, shape, Gram stain and oxidase are complementary tests used in the three cases. For V. cholerae the string test and the serologic agglutination test for O1and O139 antigens are also commonly used
References
Baumann, P & Schubert, R.H. (1994) Family II Vibrionaceae Veron. In: Krieg, N.R. & Holt, J.G. (eds). Bergey’s Manual of Systematic Bacteriology. 1st edition. Volume 1. Baltimore: Williams & Wilkins. pp. 516–517.
Farmer III, J.J., Janda, J.M., Brenner, F.W., Cameron, D.N. & Birkhead, K.M. (2005) Genus I. Vibrio Pacini 1854. In: Brenner, D.J., Krieg, N.R. & Staley, J.T. (eds). Bergey’s Manual of Sys-tematic Bacteriology. Volume 2. 2nd edition. New York, Springer Science+Business Media Inc. pp. 494–546.
ICMSF (International Commission on Microbiological Specifica-tions for Foods) (2002) Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. New York, Kluwer Academic/Plenum Publishers.
International Organization for Standardization (2008) ISO 21872-1:2007/Cor.1:2008. Microbiology of food and animal feeding stuffs – Horizontal method for the detection of potentially enteropathogenic Vibrio spp – Part 1: Detection of Vibrio parahaemolyticus and Vibrio cholerae. 1st edition: 2007, Technical corrigendum 1:2008. Geneva, ISO.
Janda, J.M. (2005) Genus XXVII Plesiomonas Habs and Schubert. In: Brenner, D.J., Krieg, N.R. & Staley, J.T. (eds). Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd edition. New York, Springer Science+Business Media Inc. pp. 740–744.
Jones, J.L. (2012a) Vibrio cholerae seogroups O1 and O139. In: Lampel, K.A., Al-Khaldi, S. & Cahill, S.M. (eds) Bad Bug Book – Foodborne Pathogenic Microorganisms and Natural Toxins Handbook. 2nd edition. [Online] Food and Drug Administration, Center for Food Safety & Applied Nutrition. Available from: http://www.fda.gov/food/foodsafety/foodborneillness/foodbor-neillnessfoodbornepathogensnaturaltoxins/badbugbook/default. htm [accessed 10th July 2012]. pp. 38–41.
Jones, J.L. (2012b) Vibrio parahaemolyticus. In: Lampel, K.A., Al-Khaldi, S. & Cahill, S.M. (eds) Bad Bug Book – Foodborne Patho-genic Microorganisms and Natural Toxins Handbook. 2nd edition. [Online] Food and Drug Administration, Center for Food Safety & Applied Nutrition. Available from: http://www.fda.gov/food/foodsafety/foodborneillness/foodborneillnessfoodbornepatho-gensnaturaltoxins/badbugbook/default.htm [accessed 10th July 2012]. pp. 29–32.
Jones, J.L. (2012c) Vibrio vulnificus. In: Lampel, K.A., Al-Khaldi, S. & Cahill, S.M. (eds) Bad Bug Book – Foodborne Pathogenic Microorganisms and Natural Toxins Handbook. 2nd edition. [Online] Food and Drug Administration, Center for Food Safety & Applied Nutrition. Available from: http://www.fda.gov/food/foodsafety/foodborneillness/foodborneillnessfoodbornepatho-gensnaturaltoxins/badbugbook/default.htm [accessed 10th July 2012]. pp. 45–48.
Kaysner, C.A & DePaola Jr., A. (2001) Vibrio. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 40, pp. 405–420.
Kaysner, C.A & DePaola Jr., A. (2004) Vibrio. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 5. [Online] Silver Spring, Food and Drug Administration. Available from: http://www. fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm070830.htm [Accessed 3rd November 2011].
Kourany, M. (1983) Medium for isolation and differentiation of Vibrio parahaemolyticus and Vibrio alginolyticus. Applied and Environmental Microbiology, 45(1), 310–312.
Martin-Carnahan, A. & Joseph, S.W. (2005) Genus I Aeromonas Stanier. In: Brenner, D.J., Krieg, N.R. & Staley, J.T. (eds). Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd edition. New York, Springer Science+Business Media Inc. pp. 557–578.
Vanderzant, C. & Splittstoesser, D.F. (eds) (1992) Compendium of Methods for the Microbiological Examination of Foods. 3rd edition. Washington, American Public Health Association (APHA).
WHO (World Health Organization) (2011) Risk assessment of Vibrio parahaemolyticus in seafood – interpretative summary and technical report, Microbiological Risk Assessment Series, N° 16. [Online] Available from: http://www.who.int/foodsafety/pub-lications/micro/MRA_16_JEMRA.pdf [Accessed 20th August 2012].
WHO (World Health Organization) (2005) Risk assessment of choleragenic Vibrio cholerae 01 and 0139 in warm-water shrimp in international trade: interpretative summary and technical report, Microbiological Risk Assessment Series, N° 9. [Online] Available from: http://www.who.int/foodsafety/publications/micro/mra9.pdf [Accessed 20th August 2012].

|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
المجمع العلمي يكرّم مجموعة من أيتام مؤسسة التكامل والتكافل الخيرية في كربلاء
|
|
|