


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Method APHA 2001 for commercial sterility or cause of spoilage of acid canned foods |
|
|
|
Read More
Date: 9-3-2016
Date: 7-3-2016
Date: 7-3-2016
|
Method APHA 2001 for commercial sterility or cause of spoilage of acid canned foods
Method of the American Public Health Association (APHA), as described in the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Deibel and Jantschke, 2001, Denny and Parkinson, 2001).
Observation: Differently from this Manual, the Compendium differentiates the commercial sterility test (chapter 61) from the test for the determination of the cause of deterioration. The decision to use the same procedure for the two tests is based on several other references, such as the Bacteriological Analytical Manual (BAM Online) of the Food and Drug Administration (Landry et al., 2001) and the Compendium of Analytical Methods of the Government of Canada, Health Products and Food Branch (MFHPB, 2001). In the Compendium, the two tests also use basically the same procedure.
1. Material required for analysis
• Thermoacidurans Broth (TAB)
• Malt Extract Broth (MEB)
• Thermoacidurans Agar (TAA)
• All Purpose Tween (APT) Broth or De Man Rogosa & Sharpe (MRS) Broth or Orange Serum Broth (OSB) (optional for lactic acid bacteria detection)
• Potato Dextrose Agar (PDA) (acidified or with antibiotics)
• Malt Extract Agar with antibiotics (optional)
• All Purpose Tween (APT) Agar or De Man Rogosa & Sharpe (MRS) Agar or u Orange Serum (OSA)
(optional for lactic acid bacteria detection)
• Dextrose Tryptone Agar (DTA)
• Agar Plug (Agar 2%) or Vaspar (vaseline:paraffin 1:1)
• Gram Stain Reagents
• Alcoholic solution of iodine
• Sanitary (bacteriological) can opener sterilized
• Sterilized scissors, tweezers, spatulas, hobby knives
• Laboratory incubator set to 30°C
• Laboratory incubator set to 35°C
• Laboratory incubator set to 55°C
2. Procedure
The scheme of analysis for testing commercial sterility or cause of spoilage of acid canned foods using the method APHA 2001 is shown in Figure 1.
a) Precautionary measures to avoid accidental contamination of the sample in the laboratory: Follow the same guidelines as those given for the test for low acid foods.
b) Preparation of the culture media: The Compendium (Deibel & Jantschke, 2001) recommends that all media to be used in the commercial sterility test of acid foods be acid, since only microorganisms capable of growing under acidic conditions would be of relevance in these products. The Compendium of Canada (MFHPB, 2001) is more explicit, recommending that the pH of the media be adjusted to the pH of the product to be analyzed. In laboratory routine, in which the media are prepared on beforehand, it may be difficult to wait until the moment the products arrived for only then to adjust the pH of the media. In this case, a viable alternative consists in preparing the media with a pH value adjusted close to the limit that separates acid foods from low acid foods (4.5). In the case of media that normally already have a pH below this value the original pH should be kept unchanged.
c) Maintenance and pre-incubation of the samples before analysis: For samples of recently processed foods (less than 30 days from the date of manufacture), pre-incubate at 25–30°C for ten days, prior to analysis. Samples intended to be stored at temperatures exceeding 40ºC (“hot vending”) should further be incubated at 55ºC for five to seven days. For all other samples, follow the same guidelines as those provided for low acid foods.
d) Aseptic opening of the packages: Follow the same guidelines as those given for low acid foods.
e) Inoculation: Homogenize the content of the pack-aging and, before inoculation, withdraw 50 g or 50 ml of the sample and transfer to sterile flasks with screw caps. Preserve this portion under refrigeration, as counter-sample.
Inoculate 1–2 g or ml of the sample in one of the media below:
4 tubes of Thermoacidurans Broth (TAB) (from two of the four tubes exhaust the oxygen before inoculating and stratify with Plug Agar or Vaspar after the inoculation).
2 tubes of Malt Extract Broth (MEB).
2 tubes of All Purpose Tween (APT) Broth or De Man Rogosa & Sharpe (MRS) Broth or Orange Serum Broth (OSB) (optional for lactic acid bacteria detection).
Note e.1) The Compendium does not include the tubes of Malt Extract Broth in the procedure, inoculating the sample directly onto two plates of Potato Dextrose Agar (PDA) acidified. How-ever, the broth was included as described by Bacteriological Analytical Manual (Landry et al., 2001) to allow inoculating a greater quantity of sample.
Note e.2) The Compendium also allows performing other inoculations directly on plates: The four tubes containing TAB may be replaced by six plates with Thermoacidurans Agar (TAA); two plates incubated aerobically at 30 or 35°C/2–5 days, two plates incubated anaerobically at 30 or 35°C/2–5 days and two plates incubated aerobically at 55°C/ 2–5 days. The two tubes containing broth APT, MRS or OSB may be replaced by two plates with agar APT, MRS or OSA incubated at 30°C/2–5 days. To inoculate the sample directly on plates streak a loopful onto each plate. If the product is liquid, it is also possible to inoculate 1 ml by pour plate technique. Incubation is conducted under the same conditions as those indicated for the tubes.
Note e.3) Sterilize the remaining content of the sample in the packages at 121ºC/30 min and discard. Wash and store the packages for future physical analyses, if necessary.
f ) Incubation: Incubate the tubes under the conditions described below. If the product is not intended to be stored at a temperature higher than 40ºC, the tubes incubated at 55ºC may be omitted. If the product has a water activity smaller than 0.85, only inoculation in PDA is necessary and the remaining media may be omitted.
2 tubes of TAB with Agar plug at 30 or 35ºC/2 to 5 days in normal atmosphere (for anaerobic and facultative anaerobic mesophilic acidophilic bacteria).
2 tubes of TAB without Agar plug at 55ºC/2 to 5 days in normal atmosphere (for aerobic thermophilic acidophilic bacteria).
2 tubes of Malt Extract Broth at 25 or 30ºC/2 to
5 days in normal atmosphere (for yeasts and molds).
2 tubes of APT, MRS or OSB at 30ºC/2 to 5 days in normal atmosphere (for lactic acid bacteria).
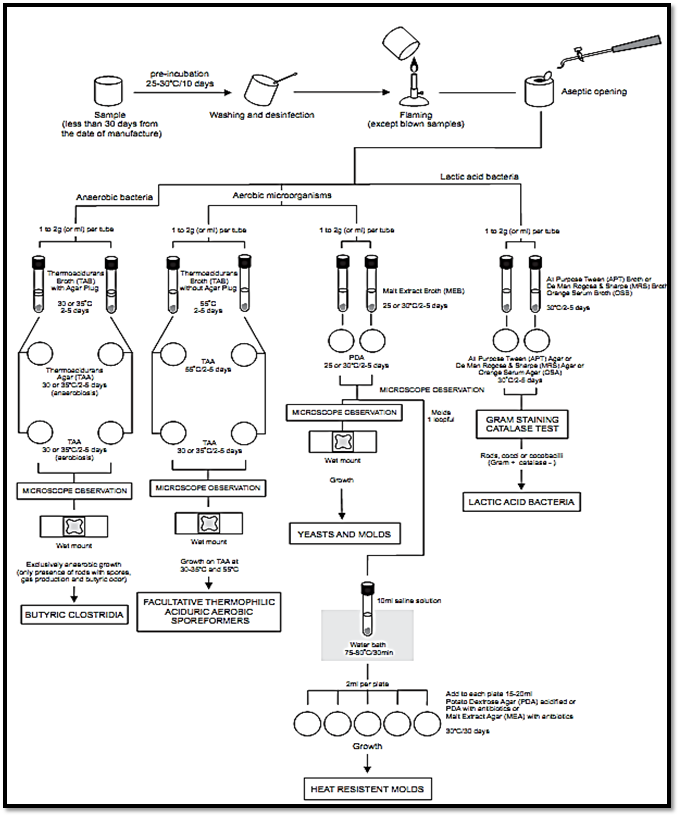
Figure 1. Scheme of analysis for testing commercial sterility or cause of spoilage of acid canned foods using the method APHA 2001 (Deibel and Jantschke, 2001, Denny and Parkinson, 2001).
g) Characterization of the microorganisms isolated in TAB at 30/35°C with agar plug: If there is growth in the tubes, subject the cultures to characterization. In case of doubt as to the development or not of microorganisms (doubtful growth), continue the test normally and, if there is no growth in the subcultures, assume that there was no growth in the original tube.
Streak a loopful of each tube onto two plates containing Thermoacidurans Agar (TAA). Of each pair of plates, incubate one under anaerobic conditions and one under aerobic conditions, for two to five days, at the same temperature of the original tube. Exclusive growth on the anaerobic plate indicates a strictly anaerobic culture. Growth on both plates (aerobic and anaerobic) indicates a facultative anaerobic culture or a mixture of facultative anaerobes and strictly anaerobes. Observe whether there is production of gas in the tubes and whether there is development of butyric odor in the tubes and on the plates.
From each TAA plate exhibiting growth prepare a smear or wet mount of the cultures, for Gram staining or fresh microscopic observation. Select for characterization a colony of each type present. Observe the morphological type(s) of the cells, which may be rods, cocci, coccobacilli, yeasts or molds. If there are rod-shaped cultures, record whether there is spore formation.
h) Characterization of the microorganisms isolated in TAB at 55°C without agar plug: In case of growth in the tubes, subject the cultures to characterization. If there is any doubt as to the development of microorganisms (doubtful growth), continue the test normally and, if there is no growth in the subcultures, assume that there was no growth in the original tube.
Streak a loopful of each tube on two plates containing Thermoacidurans Agar (TAA). Of each pair of plates, incubate one at 30 or 35°C/2–5 days and one at 55°C/2–5 days. Exclusive growth on the plate incubated at 55°C indicates a strictly thermophilic culture. Growth on both plates indicates either a facultative thermophilic culture or a mix-ture of facultative and strictly thermophiles.
From each TAA plate exhibiting growth prepare a smear or wet mount of the culture for Gram staining or fresh microscopic observation (procedures described in Chapter 5). Select for characterization one colony of each type present. Observe the morphological type(s) of the cells, which may be rods, cocci, coccobacilli, yeasts or molds.
If necessary to confirm the presence of Bacillus coagulans, the rod-shaped cultures may be inoculated onto two plates of Dextrose Tryptone Agar (DTA). Incubate one plate at 30 or 35°C/72 h and the other plate at 55°C/72 h. The occurrence of growth on the two plates, accompanied by an acid color change of the indicator (a yellow halo sur-rounding the colonies) is confirmative for the presence of B. coagulans. i) Characterization of the microorganisms isolated in Malt Extrac Broth at 25 or 30°C: If there is growth in the tubes, subject the cultures to characterization. In case of doubt as to the development or not of microorganisms (doubtful growth), continue the test normally and, if there is no growth in the subcultures, assume that there was no growth in the original tube.
Streak a loopful of each tube onto a plate containing Potato Dextrose Agar (PDA) with antibiotics. Incubate the plates at 25 or 30°C for two to five days and observe the development of cotton-like colonies, typical of molds, or non-cotton-like colonies, which are presumptive for yeasts. If there are molds present, verify the heat resistance: suspend a loopful of each colony in tubes containing 10 ml of a sterile saline solution. Trans-fer the tubes to a controlled-temperature water bath set to a temperature between 75 and 80°C and keep in the bath for 30 min, making sure that the sur-face of the liquid remains below the water surface of the bath. Distribute the 10 ml over five empty plates (2 ml/plate) and add to each plate 15–20 ml Potato Dextrose Agar (PDA) with antibiotics or Malt Extract Agar (MEA) with antibiotics. Place the plates inside a sterile plastic bag, close the bag well (to avoid drying out of the culture medium) and incubate at 30ºC for up to 30 day. Examine every week for the development of heat resistant mold colonies.
If there are presumptive yeasts colonies present prepare a smear or a wet mount of the culture obtained in each plate, for Gram staining or fresh microscopic observation. Observe the morphological type(s) of the cells, which may be yeasts or bacteria (rods, cocci, coccobacilli).
j) Characterization of the microorganisms isolated in APT/MRS/OSB: If there is growth in the tubes, subject the cultures to characterization. In case of doubt as to the development or not of microorganisms (doubtful growth), continue the test normally and, if there is no growth in the sub-cultures, assume that there was no growth in the original tube.
Streak a loopful of each tube on a plate containing either APT Agar, MRS or OSA. Incubate at 30°C for two to five days, under a microaerophilic atmosphere. From each plate exhibiting growth subject at least five colonies to the catalase test and Gram staining. The observation of Gram-positive, catalase-negative rods, cocci or coccobacilli, is confirmative of the presence of lactic bacteria.
3. Interpretation of the results
Interpretation depends on the occurrence or not of growth, the media in which and the incubation conditions under which growth occurred and whether or not the sample showed evidence of alteration.
a) Absence of growth
In normal samples, without evidence of any alteration, the absence of growth in any of the inoculated tubes indicates that the product is commercially sterile.
In samples showing evidence of alteration, the absence of growth in any of the inoculated tubes may indicate non-microbial causes of alterations or loss of viability of the culture after growth and alteration of the product. This is common, for instance, with lactic acid bacteria.
b) Growth
In apparently normal samples, without signs or evidence of alteration, the occurrence of growth in one or more inoculated tubes may indicate accidental contamination. The following situations are suspected to be caused by accidental contamination: a) Growth in only one tube of the duplicate.
b) Microflora with different characteristics in each tube of the duplicate. c) Anaerobic growth with gas production in TAA at 30–35°C, when the sample did not present any problem of blowing of vacuum loss during the pre-incubation step. d) When observing the sample directly through the micro-scope, find different morphological types from those found in the cultures. In such cases, repeat the test, using the stored counter-sample, observing strictly the precautionary care to avoid accidental contamination of the sample in the laboratory.
If there is no indication of accidental contamination, interpret the results in accordance with the guidelines of Table 1 and the items described below:
In altered samples, the occurrence of growth is expected. Interpret the results in accordance with the guidelines given in Table 1 and the items below:
c) Growth at 30–35°C in TAB with Agar plug → TAA anaerobiosis: The objective of the anaerobic incubation of TAB or TAA at 30–35°C is to verify the presence of anaerobic mesophilic spore forming aciduric bacteria (butyric clostridia). However, several other microorganisms may grow under these conditions:
c.1) Mixed microflora: The growth of a mixed microflora with different morphological types (rods, cocci, coccobacilli, yeasts, molds) reveals the presence of one or more microbial groups that do not form spores and that do not resist thermal processing of acid products. This indicates that the product is not commercially sterile and leakage is the probable cause. Verify the presence of points of leakage in the packaging to confirm the diagnosis.
c.2) Non-spore forming culture: The growth of only one morphological type of microorganism that does not form spores (only molds, only yeasts, only cocci or only coccobacilli) reveals the presence of a microflora that does not resist thermal processing of acid products. This indicates that that the product is not commercially sterile and leakage is the probable cause. Verify the presence of points of leakage in the packaging to confirm the diagnosis.
c.3) Culture of only rods: The interpretation will depend on the production of spores and the requirement of oxygen for growth (observed on the TAA plates incubated at 30–35°C under aerobic and anaerobic conditions).
Table 1 Keys to probable cause of spoilage in acid or acidified canned foods (Denny and Parkinson, 2001).
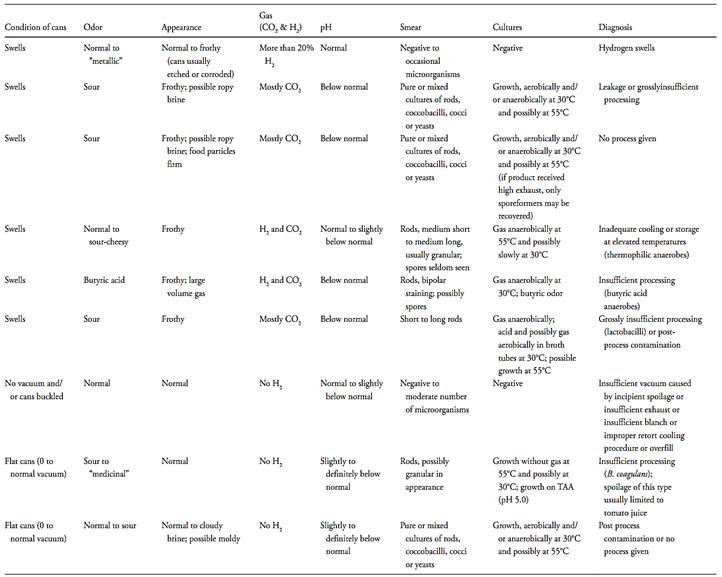
c.3.1) exclusively anaerobic growth with spore formation: The growth of spore-forming, Gram-positive rods under exclusively anaerobic conditions, with the production of gas (in TAB) and the development of a butyric odor (in TAB or on TAA) confirms the presence of anaerobic mesophilic aciduric spore-forming bacteria (butyric clostridia). This indicates that the product is not commercially sterile and underprocessing is the probable cause.
c.3.2) Aerobic and anaerobic growth: Reveals the presence of facultative anaerobes or a mixture of strictly and facultative anaerobes. If there are any spores, this indicates that the product is not commercially sterile and underprocessing is the probable cause.
When there are no spores, it indicates leakage (verify the presence of points of leakage in the packaging to confirm the diagnosis).
d) Growth in TAB → TAA at 55°C: The objective of incubation of TAB or TAA at 55°C is to verify the presence of thermophilic, aciduric, spore forming bacteria, particularly B. coagulans. The occurrence of growth on the DTA plates at 30–35 and 55°, accompanied by acid color change of the indica-tor (a yellow halo surrounding the colonies) con-firms the presence of B. coagulans, indicating that the product is not commercially sterile and under-processing is the probable cause. Alicyclobacillus can also develop in TAB or on TAA, but generally does not grow on DTA. Exclusive growth of strictly thermophiles in products that do not show any evidence of alteration and that are not intended for storage at high temperatures still indicates commercial sterility. If the product shows sign of having been altered, the presence of this group indicates spoilage most probably caused by slow cooling and/or storage at high temperatures.
e) Growth in Malt Extract Broth → PDA: The objective of inoculation of Malt Extract Broth or PDA is to detect the presence of yeasts and molds, particularly heat-resistant molds. The growth of heat-resistant molds indicates that the product is not commercially sterile and underprocessing is the probable cause. The growth of non-heat-resistant
molds or yeasts indicates that the product is not commercially sterile and leakage is the probable cause. Verify the presence of points of leakage in the packaging to confirm the diagnosis.
f ) Growth in APT, MRS, OSB Broth → APT, MRS or OSA Agar: The objective of inoculation of APT, MRS or OSB is to verify the presence of lactic bacteria, which are confirmed by the observation of Gram-positive, catalase-negative and non-spore-forming rods, cocci or coccobacilli. This indicates that the product is not commercially sterile and leakage or gross underprocessing are the probable cause. Verify the presence of points of leakage in the packaging to confirm the diagnosis.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Deibel, K.E. & Jantschke, M. (2001) Canned foods – tests for commercial sterility In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 61, pp. 577–582.
Denny, C.B. & Parkinson, N.G. (2001) Canned foods – Tests for cause of spoilage. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 62, pp. 583–600.
Landry, W.L., Schwab, A.H. & Lancette, G.A. (2001) Examination of Canned Foods. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 21A. [Online] Silver Spring, Food and Drug Administration. Available from: http://www.fda.gov/Food/Sci-enceResearch/LaboratoryMethods/BacteriologicalAnalytical-ManualBAM/ucm109398.htm [Accessed 10th November 2011].
Ashton, D. & Bernard, D.T. (2001) Thermophilic anaerobic spore formers. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 26, pp. 249–252.

|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
ندوة ثقافية حول آفة المخدرات وطرق مكافحتها
|
|
|
|
الأمانة العامة للعتبة الكاظمية المقدسة تعقد ندوة صحية عن الكشف المبكر لسرطان الثدي
|
|
|
|
رئيس الشؤون الدينية التركي يتشرف بزيارة الإمامين الكاظمين "عليهما السلام"
|
|
|
|
قسم الشؤون الفكرية يقيم برنامج (صنّاع المحتوى الهادف) لوفدٍ من محافظة ذي قار
|