


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 27-7-2016
Date: 18-1-2017
Date: 28-12-2020
|
Artificial Extracellular Matrices
It will be extremely challenging to reproduce exactly a biological extracellular matrix through artificial means in the coming decades. To obtain a scaffold resembling biological ECM, many researchers currently use Matrigel, a cocktail of substances extracted from natural ECM. The advantage of Matrigel is that it is a liquid at temperatures below 4C, where it can be mixed with desired cell populations to result in a pre-seeded ready-to-implant scaffold upon gelation at physiological temperatures. Despite the superior attributes of ECM, it is sometimes advised to use artificial scaffolds for tissue engineering to achieve reproducibility and control of the individual parameters.
A protocol for the manufacture of a so-called ideal scaffold has yet to be developed. Therefore, special attention must be paid to the essential
Table1: Ideal parameters for tissue scaffolds.
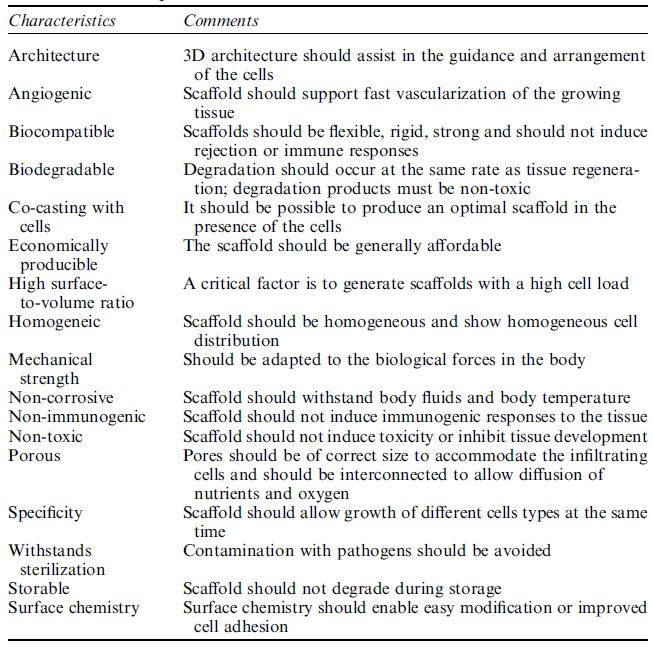
parameters of ECM, which are to be mimicked. Irrespective of the application, the scaffold should (i) be bio-compatible, (ii) provide a 3D template for guided growth, (iii) have a highly porous structure for maximum cell load, nutrition and oxygen diffusion, (iv) degradation dynamics should match de novo ECM synthesis, (v) be mechanically resistant to biological forces, (vi) withstand sterilization and (vii) production should be economically feasible (Table ). The shape of the starting material, including the fibers, microspheres and sheets and films, must be determined and the generation of the scaffold microstructure studied. For this purpose, different materials, including ceramics, polymers and composites, are under investigation. Ceramics such as hydroxyapatite, tricalcium phosphate, glass ceramics and glass are generally used for the reconstruction of hard tissue, whereas polymers are implemented for the formation of soft tissues. Polymers are usually the material of choice as their properties can be modulated with ease. Gelatin, elastin, collagen, fibrin glue and hyaluronic acid, all of biological origin, are the most important polymers for production of artificial scaffolds. Synthetic polymers are also available including poly(lactic acid), poly(glycolic acid), polycaprolactone poly(lactic-co-glycolic acid), polyethylene, poly(ethylene glycol), poly(ethylene oxide)-block-polycaprolactone, poly(ethylene terephthalate), polytetrafluoroethylene and polyurethane. Both degradable and non-degradable synthetic polymers exist. Although degradable polymers are usually selected, such as the most widely used poly(lactic acid), the degradation products must be either biologically inert or easily metabolized, but above all non-toxic.
As a result of refining the production process for scaffold material, the spectrum and quality of scaffold types have increased considerably. Latest-generation scaffolds have average pore sizes ranging from a few nanometers to several micrometers. In many cases the pores are interconnected to allow the transport of nutrients and metabolic waste and to provide a high surface-to-mass ratio for the promotion of cell differentiation and proliferation. These scaffolds are produced by various techniques, including (i) electrospinning, (ii) phase separation,(iii) foaming processes, (iv) microsphere sintering, (v) solid free-form fabrication, (vi) shape deposition manufacturing, (vii) fused deposition modeling, (viii) non-fused liquid deposition modeling, (ix) 3D printing,(x) selective laser sintering, (xi) stereo-lithographic processes, (xii)
leaching processes and (xiii) self-assembly. Electrospinning is one of the most commonly used techniques to develop scaffolds for tissue engineering, because it is possible to modulate the fiber size from the nanometer to the micrometer scale and to guide the arrangement of fibers in the non-woven 3D fiber network. The process consists of forcing a polymer melt through an electrically charged nozzle (10–20 kV), thereby forming a thin polymer string. The solvent quickly evaporates en route to the collector, which can be a simple grounded plate for random orientation of the fibers or a drum for their parallel alignment. Different processing parameters such as the polymer or solvent chosen, viscosity, surface tension, the charge applied, the polymer mass flux through the nozzle, the processing temperature and the humidity, all control the diameter and the ultrastructure of the generated fibers. Another advantage of electrospinning is that fibers of different polymers can be co-deposited into a multilayered structure. Such scaffolds display a range of favorable properties for cell attachment (e.g. a wide range of pore sizes, high porosity and parallel fiber orientation). Yang et al. showed that cells tend to grow in the direction of the fiber and that spatial orientation of the fibers therefore plays a key role in guiding cell growth and the subsequent organization of the tissues.
Phase separation is another valuable and cost-effective process to form scaffolds for tissue engineering. The polymer is usually dissolved in a solvent that is brought into contact with a non-solvent. During the casting process, the solvent diffuses out and the non-solvent diffuses into the polymer solution until the polymer becomes unstable and precipitates, thereby forming the final scaffold after a only a few hundred milliseconds. The pore size of such scaffolds can be varied from nanometers to several micrometers and can be extended by other processes such as salt leaching. These scaffolds are ideal for the engineering of special tissues such as skin and bone, the pore size of which must be in the range 0.04–0.4 mm.
Self-assembly systems are gaining in importance in the development of artificial scaffolds. They utilize a bottom-up approach, whereby molecules undergo self-association to form a higher order structure without external manipulation. Special attention should be paid to the monomer, which must be well designed to induce chemical reactivity and structural compatibility. Given these prerequisites, various building blocks have been used, such as nanofiber scaffolds, peptides and dipolar molecules. Although the design of scaffolds by molecular selfassembly is extremely challenging, it is a promising process that can be performed under physiological conditions which allow pre-seeding of the scaffolds with desired cell populations.
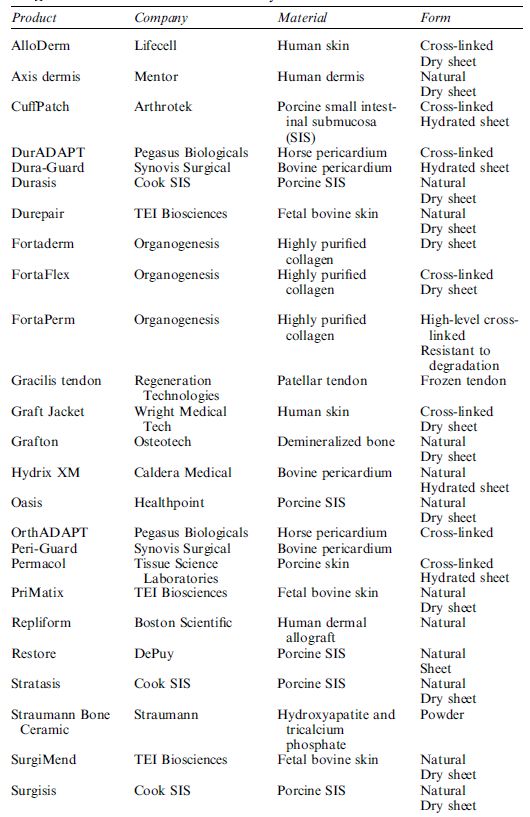
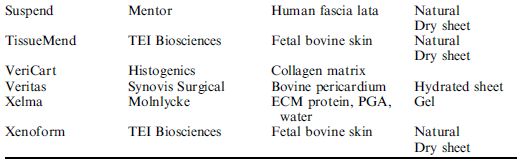
The choice of a specific scaffold for tissue engineering is usually based on the type of tissue to be engineered. Several researchers have provided specific information concerning each type of scaffold and their application. However, this information should be treated with caution because the described scaffolds were, in part, developed by material scientists and chemists and have not been tested in depth with cell cultures. For example, foaming processes may result in inclusion of air bubbles which compromise cell colonization, salt-leaching processes do not completely remove entrapped salt crystals and sterilization radically changes the microstructure of polymer scaffolds. In conclusion, only successful testing in in vivo models will finally prove whether a scaffold is suitable for tissue engineering or not. Despite all the obstacles that scientists have encountered in producing natural or synthetic scaffolds for tissue engineering, many scaffolds have already been approved by the FDA and are being used in a clinical setting (Table 2).
Table 2 A selection of commercially available scaffolds in 2005.

|
|
|
|
اكتشاف تأثير صحي مزدوج لتلوث الهواء على البالغين في منتصف العمر
|
|
|
|
|
|
|
زهور برية شائعة لتر ميم الأعصاب التالفة
|
|
|
|
|
|
جمعيّة العميد وقسم الشؤون الفكريّة تدعوان الباحثين للمشاركة في الملتقى العلمي الوطني الأوّل
|
|
|
|
الأمين العام المساعد لجامعة الدول العربية السابق: جناح جمعية العميد في معرض تونس ثمين بإصداراته
|
|
|
|
المجمع العلمي يستأنف فعاليات محفل منابر النور في واسط
|
|
|
|
برعاية العتبة العباسيّة المقدّسة فرقة العبّاس (عليه السلام) تُقيم معرضًا يوثّق انتصاراتها في قرية البشير بمحافظة كركوك
|