


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-10-2019
Date: 14-10-2019
Date: 3-11-2019
|
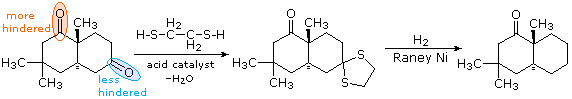
Hydrogenolysis of Thioacetals
In contrast to the previous two procedures, this method of carbonyl deoxygenation requires two separate steps. It does, however, avoid treatment with strong base or acid. The first step is to convert the aldehyde or ketone into a thioacetal, as described earlier. These derivatives may be isolated and purified before continuing the reduction. The second step involves refluxing an acetone solution of the thioacetal over a reactive nickel catalyst, called Raney Nickel. All carbon-sulfur bonds undergo hydrogenolysis (the C–S bonds are broken by addition of hydrogen). In the following example, 1,2-ethanedithiol is used for preparing the thioacetal intermediate, because of the high yield this reactant usually affords. The bicyclic compound shown here has two carbonyl groups, one of which is sterically hindered (circled in orange). Consequently, a mono-thioacetal is easily prepared from the less-hindered ketone, and this is reduced without changing the remaining carbonyl function.



|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تجري القرعة الخاصة بأداء مناسك الحج لمنتسبيها
|
|
|