

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Interactions between Particles
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 75
9-7-2017
2136
Interactions between Particles
Since chemical species in condensed phases interact strongly, solids and liquids are more complicated than gases, where intermolecular forces are usually negligible. The interactions of atoms, molecules, and ions are electrostatic in origin, but there are several types of interaction with quite different energies: Ion-ion interactions involve by far the largest energies. When like charges interact (a repulsive interaction), the energy is positive. When unlike charges interact (an attractive interaction), the energy is negative. These interactions are most important in ionic crystals.
Ion-dipole interactions are next in order of interaction energy. Such interactions are exemplified by the solvation of ions such as Na+ and Cl- by water. Water has a large dipole moment and H2O molecules are strongly attracted to Na+ with the negative end (O) of the water dipoles oriented toward the cation. Similarly Cl- is solvated with the positive end of the water dipole (H) oriented toward the anion. Dipole-dipole interactions are responsible for the cohesion of dipolar liquids. For example, methyl ether, CH3OCH3, with a dipole moment, boils at -25°C, but propane, CH3CH2CH3, with about the same molar mass but negligible dipole moment, boils at - 45°C.
Dipole–induced dipole interactions are also important. Nonpolar molecules tend to have their electron clouds attracted (or repelled) by a nearby dipolar molecule oriented with its positive (or negative) end toward the nonpolar species. This induced dipole interacts, on the average, with the dipolar molecule as if it were a permanent dipole.
This effect is responsible for the solubility of nonpolar gases such as O2, N2, or CO2 in water. Induced dipole–induced dipole interactions, also called London forces, result when a nonpolar molecule undergoes a distortion, perhaps as a result of a collision, which results in a momentary separation of the centers of positive and negative charge. The resulting dipole induces a dipole in a neighboring molecule, resulting in an attractive interaction.
Both dipole–induced dipole and induced dipole-induced dipole interactions generally increase with number of electrons, particularly in atoms on the outer surface of the molecule. For example, the boiling points of the inert gases—He (4 K), Ne (27 K), Ar (88 K), Kr (120 K), and Xe (166 K)—increase with the number of polarizable electrons. UF6 (bp 56°C) is more volatile than SbCl5 (bp 79°C); uranium has more electrons than antimony, but the halogens are on the surface of the molecules, and there are more polarizable electrons in the five Cl atoms than in the six F atoms. Dipole-dipole, dipole–induced dipole, and induced dipole–induced dipole interactions are often referred to collectively as van der Waals interactions.
Some dipolar molecules exhibit much greater intermolecular interactions than might have been expected from their dipole moments alone. This effect is illustrated by the data of Table 1.1.
Table 1.1. Boiling Points of Some Simple Hydrides
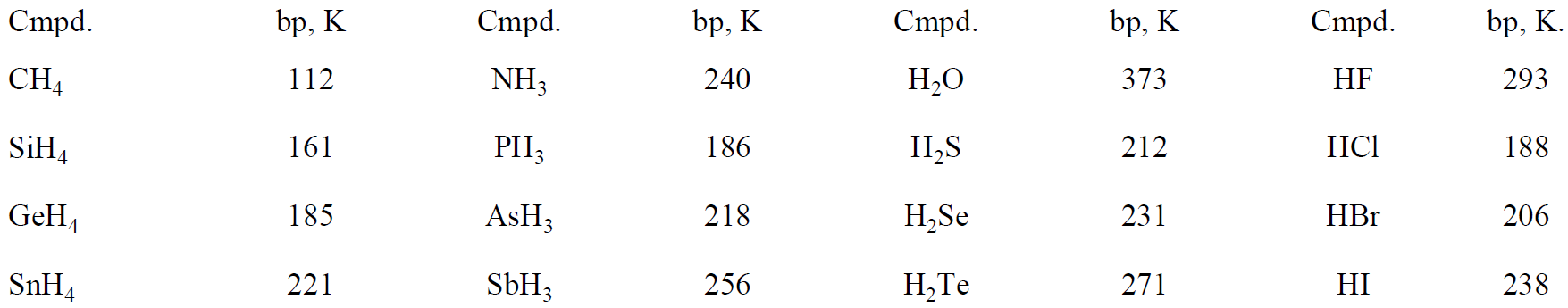
The heavier hydrides behave as expected, i.e., an increase in boiling point with number of polarizable electrons. However, NH3, H2O, and HF have anomalously high boiling points. This effect is due to hydrogen bonding, the tendency of hydrogen atoms bound to electronegative atoms to interact with unshared electron pairs on adjacent molecules.
Hydrogen bonding is expected for hydrides with a strongly electronegative central atom—N, O, and F—with unshared pairs of electrons which can interact with the positive hydrogen of an adjacent molecule. Water is by far the best former of hydrogen bonds since it has two unshared pairs and two H's. NH3 (one unshared pair and three H's) and HF (three unshared pairs and one H) have only half the potential hydrogen bonds of H2O.
Hydrogen bonds are not limited to NH3, H2O, and HF. Molecules containing NH2, -OH, or -F groups are capable of hydrogen bonding. Hydrogen bonds are of great importance in many chemical and biochemical systems. The structure of ice depends on hydrogen bonds; the base pairing scheme of DNA vital to the genetic code relies on hydrogen bonds; the structures of proteins and many synthetic polymers are dictated by hydrogen bond formation.
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















