
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Atomic Constituents Electrons and Nuclei
المؤلف:
Roger J Blin-Stoyle, FRS
المصدر:
Physics of Particles, Matter and the Universe
الجزء والصفحة:
P 65
22-5-2016
2298
Atomic Constituents Electrons and Nuclei
In the last chapter the idea was introduced that an atom consists of negatively charged electrons and a positively charged nucleus such that the total negative charge of the electrons exactly balanced the positive charge of the nucleus. It is now time to explain how this idea came about. During the last half of the 19th century physicists studied the way in which electric currents flowed through gases. A gas is normally an insulator but, at a sufficiently low pressure and with a sufficiently high voltage (potential difference) between a positive and a negative electrode, a current will flow and the gas becomes luminous. Experiments of this sort are carried out in glass tubes, known as discharge tubes (see figure 1.1), and present-day ‘neon tubes’ are of this kind. At very low pressures the emitted light normally observed disappears but, if a hole is made, as shown, in the positive electrode, a glow is observed at the end of the tube. This glow can be attributed to a beam of negatively charged particles, which we now know to be electrons, being attracted from the negative towards the positive electrode. In the arrangement shown in figure 1.1 the electrons
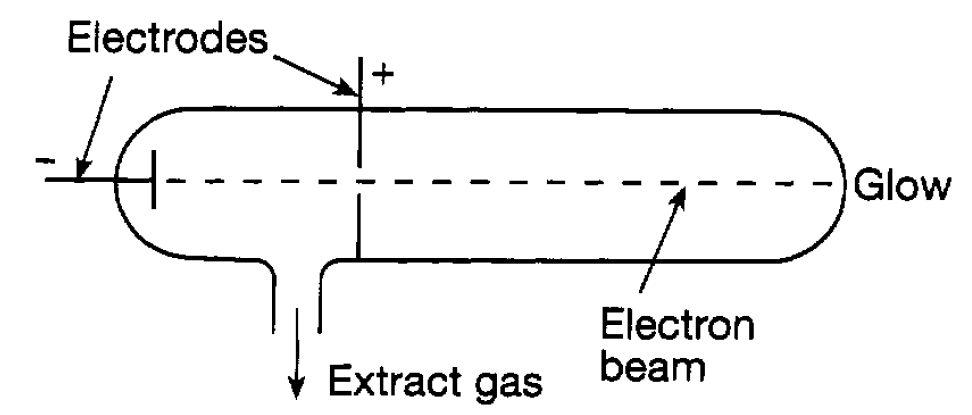
Figure 1.1: The flow of electrons through a discharge tube.
pass through a hole in the positive electrode, and finally strike the end of the tube. Where they strike the tube a glow is produced and the process is familiar in every home as being essentially responsible for television pictures. In the 1890s J. J Thomson studied this process very carefully. He applied electric and magnetic fields to the narrow beam of electrons defined by the aperture and measured how the beam moved in relation to the strength of these fields. From these measurements he was able to deduce the ratio of the charge to the mass of the electrons. If electrons are atomic constituents they can be knocked out of atoms. An atom, losing an electron, is then left with a net positive charge equal in magnitude to that of the electron. Such an entity is referred to as appositive ion. Ions can be produced in various ways, for example by knocking electrons out of an atom using x-rays. In addition, negative ions can also be formed when an extra electron attaches itself to an atom. Such ions have the property that, in a very damp atmosphere saturated with water, droplets can form on them, the water being attracted by the ionic charge. By studying the way such droplets move in an electric field it is possible to estimate the electric charge carried by a droplet, that is, the magnitude of the charge of an electron. This approach was developed particularly by R A Millikan who, in 1911, using oil drops rather than water drops, obtained an accurate value for the magnitude of the electron charge which is now known to be e = 1.602×10-19 C. Knowing the charge, Thomson’s result enables the mass of the electron to be deduced. This turns out to be 9.11 × 10-31 kg. The important thing to note at this stage is that the electron mass is some 1836 times smaller than that of the lightest atom hydrogen. So, in the light of this, we now have to consider where the rest of the atomic mass is located and also how the positive charge is distributed. We have that atoms in a solid are separated by distances of around 10-10m. In a solid the atoms are squashed together and so one can (correctly) presume that atomic sizes are of this order of magnitude, In considering the way in which atoms are constructed, Thomson initially thought that the positive charge and the bulk of the mass occupied a sphere of atomic size and that the electrons were dotted around in this sphere like currants in a plum pudding (see figure 1.2(a)). However, developments in the next few years showed that this idea was completely wrong. At the same time as Thomson was studying electrons, Becquerel and Pierre and Marie Curie in France and Rutherford in Britain were investigating the new phenomenon of radioactivity in which different forms of radiation were emitted by heavy atoms such as radium and uranium. These radiations will be. Suffice it to say here that one of them, referred to as alpha radiation, was found to consist of a stream of positively charged helium atoms (i.e. helium ions). In order to understand the details of atomic structure Rutherford suggested to two of his colleagues, Geiger and Marsden, that they should study the way these ‘alpha particles’ were scattered by the electrical force due to the positive and negative charges in an atom when they passed through a thin sheet of material. This would test whether the ‘plum pudding’ idea was correct because, if it were, the expectation was that the alpha particles would hardly deviate as they passed through the material. This experiment was carried out using a thin gold foil and studying the direction in which the alpha particles were scattered by the flashes they produced when they struck a zinc sulphide screen (see figure 1.2(b)). The flashes were due to sudden movements of electrons when ‘struck’ by alpha particles.
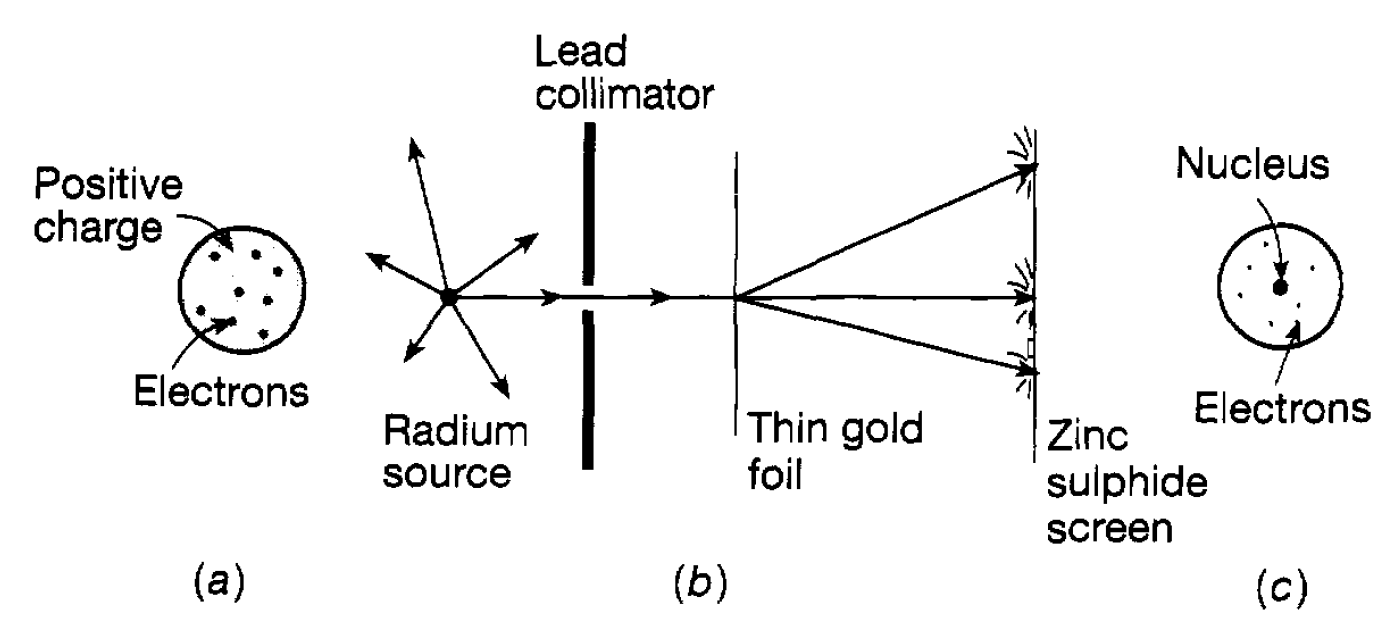
Figure 1.2: (a) The ‘plum pudding’ atom; (b) the Geiger-Marsden experiment; (c) the nuclear atom.
Although most alpha-particles were hardly scattered, a few were scattered through wide angles and even, very occasionally, bounced completely backwards. Rutherford was amazed at this latter observation and likened the result to shooting a 15in shell at a piece of tissue paper and having it coming back and hitting you. He finally concluded in 1911 that all the positive charge and the bulk of the atomic mass must be concentrated in a very small volume the atomic nucleus at the centre of an atom as in figure 1.2(c) and that the ‘bouncing back’ effect occurred when an alpha particle experienced a ‘head on’ collision with a nucleus. Detailed analysis of the scattering indicated that the size of the gold nucleus, was of the order of 10-14m, some four orders of magnitude smaller than the atomic size. Thus was established the idea of the nuclear atom in which all the positive charge and the bulk of the atomic mass is concentrated in a nucleus, whilst the negatively charged electrons surrounded the nucleus, occupying a much larger volume. The next question is, of course, what are the electrons doing in this space? This can only be answered in terms of the ideas of quantum mechanics which were emerging over this same period.
 الاكثر قراءة في الفيزياء الذرية
الاكثر قراءة في الفيزياء الذرية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















