GTP-Binding Proteins
GTP-binding proteins are members of a superfamily of regulatory proteins whose activity is controlled by binding GTP. They act as conformational molecular switches that regulate numerous and diverse processes throughout the eukaryotes. Many GTP-binding proteins are devoted to signal transduction, mediating information transfer from receptors to intracellular effector proteins. Others regulate such diverse processes as the traffic of membrane proteins and lipids via cytoplasmic transport vesicles, the cytoskeleton-mediated control of cell morphology, nuclear import/export, and such membrane-reforming processes as endocytosis, protein secretion, and budding. In their GTP-bound, active forms, GTP-binding proteins can stimulate or inhibit the activity of cellular effector proteins, recruit effectors to specific intracellular sites, or sequester effectors and thus block their activity. These proteins thus lie at the convergence of multiple regulatory pathways and function as major integrators of cellular information.
GTP-binding proteins are divided into two major classes, each of which is further divided into individual families. Generally, these structurally determined groupings also indicate related functional specialization and biochemical properties. GTP-binding proteins are ubiquitous throughout the eukaryotes, however, and structurally related proteins may perform strikingly different functions in distantly related organisms.
The two major groups of GTP-binding proteins are the heterotrimeric G proteins and the small, monomeric GTP-binding (SMG) proteins. SMGs are also commonly referred to as G proteins, or small G proteins, and the nomenclature is not well established. Heterotrimeric G proteins are composed of a GTP-binding a subunit ( to 45 kDa) and a dimer of b and g regulatory subunits 35) kDa and 7 to 8 kDa). Heterotrimeric G proteins convey signals from receptors at the cell surface to cellular effector proteins (. The SMG proteins (20 to 25 kDa) are involved in more diverse functions. In addition to these two major classes, other GTP-binding proteins with related primary structures, tertiary structures, and GTPase cycles include the elongation factors of translation (with bacterial EF-Tu as prototype) and dynamin. Heterotrimeric G proteins are grouped according to the structures and functions of their a subunits and are named with subscripts originally linked to their functions (1, 2). Members of the Gs family were the first G proteins to be discovered as stimulators (hence “s”) of adenylyl cyclase. The Gi's were initially defined as cyclase inhibitors (“i”), but they also perform many other functions. The Gq's stimulate the phosphatidylinositol bisphosphate-specific phospholipase C-b's. Functions of the G12 / 13 family include regulation of the rho SMG family and of / exchangers. The bg subunits are also diverse: There are 5 Gb subunits and at least 11 Gg subunits in mammals. While not all of the 55 conceivable Gbg dimers occur, their potential diversity is huge.
The SMG proteins cluster in five major subfamilies according to their structure and function. Smaller families also exist, and assignment of some SMGs to families remains uncertain. The ras proteins constitute a family of about five proteins in mammals, although gem/kir proteins are also assigned to this group (3-6). Ras proteins were the first SMGs to be identified, because they are active and frequently observed oncoproteins when mutated to constitutively active forms or when overexpressed (7). Ras proteins are the prototypical and best-studied examples of the SMG group . The rac family (rac, rho, CDC42) are involved in both signal transduction and in the regulation of cytoskeletal control of cell morphology (8, 9). Rab family members (at least 20) are involved in protein secretion and endocytosis (10, 11). ARFs (at least 6) mediate the budding and fusion of intracellular membrane vesicles (12, 13). Ran mediates both inward and outward traffic of macromolecules from the nucleus (14, 15).
Both heterotrimeric G proteins and SMG proteins act by traversing a tightly controlled cycle of GTP binding and hydrolysis. GTP binding activates G proteins (Fig. 1) (1, 16). Activation is terminated when the bound GTP is hydrolyzed by the G protein's intrinsic GTPase activity. The GDP-bound form is inactive, as is the unliganded form. Each step in this cycle—GTP binding, GTP hydrolysis, and GDP release—is relatively slow, and each can be regulated both positively and negatively. Activation—GDP dissociation and GTP binding—is promoted by guanine nucleotide exchange factors (GEFs). GEFs are also referred to as exchange catalysts. They can be either cell-surface receptors that act in response to extracellular signals or cytosolic proteins that are regulated by allosteric ligands or phosphorylation. Activation is inhibited by GDP dissociation inhibitors )GDIs), which are regulated independently. Deactivation is also accelerated by GTPase-activating proteins (GAPs), providing a third regulatory input. The net output represents the integration of all three inputs.
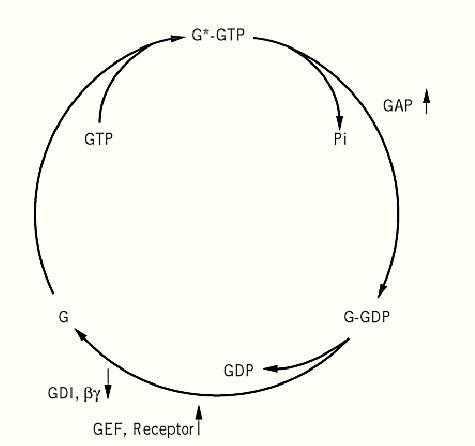
Figure 1. The regulatory GTPase cycle for GTP-binding proteins. GTP-binding proteins, G, are converted by GTP to the active state G*-GTP in which they can bind and activate effector proteins. Activation is terminated by hydrolysis of the bound GTP to GDP, which is usually a slow process that is accelerated by GTPase-activating proteins (GAPs). The inactive G–GDP complex is relatively stable, unless nucleotide exchange is catalyzed by a guanine nucleotide exchange factor (GEF). In the case of heterotrimeric G proteins, the GEFs are a family of cell-surface receptors that bind ligands on the extracellular face of the plasma membrane. The inactive, GDP-bound state is stabilized by GDP-dissociation inhibitors (GDI), which in the case of the heterotrimeric G proteins are the Gbg subunits.
The differing rates of each step in the GTPase cycle, along with their various modes of regulation, allow GTP-binding proteins to operate in one of three conceptually different switching modes:
toggles, meters, or timers. (1) A GTP-binding protein that traverses the GTPase cycle only once with respect to the process that it regulates behaves as an on/off switch. It is toggled to the active state by GEF-catalyzed activation and maintains its activity until a GAP turns it off. (2) A G protein that traverses the GTPase cycle quickly produces a graded signal in which its fractional activation, averaged over time, is a meter of the relative inputs to activation and deactivation. (3) A G protein can simply allow a process to occur for a fixed period of time, the lifetime of the bound GTP prior to its hydrolysis. While these basic patterns are formally the same, the distinctive kinetic properties of each determine how a G protein will function in the cell. The ability to work in these three different ways are vital to the functional diversity of G-protein action.
References
1. E. M. Ross (1989) Signal sorting and amplification through G protein-coupled receptors. Neuron 3, 141–152.
2. A. G. Gilman (1987) G proteins: transducers of receptor-generated signals, Annu. Rev. Biochem. 56, 615–649.
3. H. R. Bourne, D. A. Sanders, and F. McCormick (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127.
4. H. R. Bourne, D. A. Sanders, and F. McCormick (1990) The GTPase superfamily I. A conserved switch for diverse cell functions. Nature 348, 125–132.
5. G. Bollag and F. McCormick (1991) Regulators and effectors of ras proteins. Annu. Rev. Cell Biol. 7, 601–632.
6. G. M. Bokoch (1996) Interplay between Ras-related and heterotrimeric GTP binding proteins: lifestyles of the big and little. FASEB J. 10, 1290–1295.
7. M. Barbacid (1987) ras Genes. Annu. Rev. Biochem. 56, 779–827.
8. L. Van Aelst and C. D''Souza-Schorey (1997) Rho GTPases and signaling networks. Genes Dev. 11, 2295–2322.
9. A. Hall (1998) Rho GTPases and the actin cytoskeleton. Science 279, 509–514.
10. P. Novick and M. Zerial (1997) The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496–504.
11. V. M. Olkkonen and H. Stenmark (1997) Role of Rab GTPases in membrane traffic. Intl. Rev. Cytol. 176, 1–85.
12. M. G. Roth and P. C. Sternweis (1997) The role of lipid signaling in constitutive membrane traffic. Curr. Opin. Cell Biol. 9, 519–526.
13. A. L. Boman and R. A. Kahn (1995) Arf proteins: the membrane traffic police? Trends Biochem. Sci. 20, 147–150.
14. J. M. Avis and P. R. Clarke (1996) Ran, a GTPase involved in nuclear processes: its regulators and effectors. J. Cell Sci. 109, 2423–2427.
15. D. Gorlich and I. W. Mattaj (1996) Nucleocytoplasmic transport. Science 271, 1513–1518.
16. M. S. Boguski and F. McCormick (1993) Proteins regulating ras and its relatives. Nature 366, 643-654.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


