النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Disc Electrophoresis
المؤلف:
A. Chrambach
المصدر:
In The Practice of Quantitative Gel Electrophoresis
الجزء والصفحة:
21-4-2016
3251
Disc Electrophoresis
Gel electrophoresis in discontinuous buffer systems is known as “disc electrophoresis” (1). Its main advantage is the provision of a highly concentrated starting zone even when the sample is dilute. It employs at least two electrophoresis buffers, i.e., a buffer discontinuity. They differ in either a cation or anion, whereas the counterion is the same. One of the differing ions, known as the “trailing ion,” has an electrophoretic mobility less than the species of interest to be separated; the other, the “leading ion,” has a greater mobility. Embedded within such a system, the species of interest will, at steady-state, migrate within a moving boundary between the leading and trailing ions, at a field strength (conductance) and concentration that is fixed (“regulated”) by the mobilities and concentrations of the leading, trailing, and common buffer ions constituting the boundary. This “regulation” imposes a “regulated” mobility on all trailing species with net mobilities equal to, or greater than, the trailing ion; hence, the system is designated as “equal-mobility-electrophoretic” or isotachophoretic. A number of species migrating isotachophoretically in order of their net mobilities is called a “stack.” The regulation of mobilities and concentrations behind the leading ion, resulting from the requirement for electroneutrality and the conservation of mass, gives rise to an increased field strength behind the fastest migrating (leading) ion in the electric field (Fig. 1). The gradient in field strength developing behind the leading ion causes equally oriented Joule heat and pH gradients, a gradient in mobility accelerating the trailing ions until at equilibrium they have attained the net mobility of the leading ion, and a gradient of specific conductance oriented inversely to the field strength gradient (Fig. 1). The key benefit of regulation of the trailing buffer phase by the leading ion is that a macromolecular ion present in that phase will migrate within the stack at a very high concentration, estimated to be on the order of 100 mg/mL for a 100-kDa protein, independent of the degree of dilution of the macromolecule in the original sample. Therefore, very dilute samples can produce very condensed zones of sample within the gel.
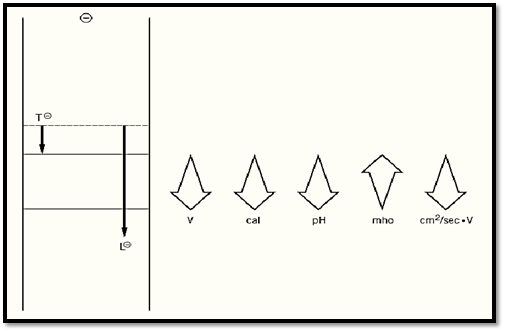
Figure 1. Operation of discontinuous buffer systems. Prior to electrophoresis, a stationary boundary (dotted line) separates the trailing ion T in the cathode buffer from the leading ion L in the gel buffer. As T and L electrophorese out of the starting zone, the greater instantaneous velocity of L would tend to create behind it an “ion vacuum.” This, however, would be in violation of the laws of conservation of mass and of the maintenance of electroneutrality. Operation of these two laws gives rise to a “regulation” in the zone behind L, which leads to an increase in field strength, Joule heat, and pH, and a decrease in conductance. These, in turn, produce an increased mobility of all species migrating between T and L until they attain the net mobility of L.
In disc electrophoresis, the concentrating (stacking) buffer phase is embedded in a gel that does not sieve but is present only to prevent convection; this gel is contiguous with a sieving gel, the “resolving gel.” As the stack of moving boundaries containing the concentrated sample enters the resolving gel, the buffer boundaries are unaffected by the elevated gel concentration and changed pH, whereas macromolecules are altered in their migration by both. The retardation due to the increased gel concentration causes the macromolecules to migrate at a mobility less than that of the trailing ion. Therefore, they “unstack” and separate according to their mobilities; in contrast, the stack of buffer ions remains (Fig. 2), forming a moving boundary “front” that can be marked by an appropriate tracking dye. The ratio of macromolecular mobilities relative to the stacked tracking dye ) in terms of relative mobility, Rf) characterizes an electrophoretic band and can be used, when measured at several different gel concentrations, to specify the size and net charge of the macromolecule.
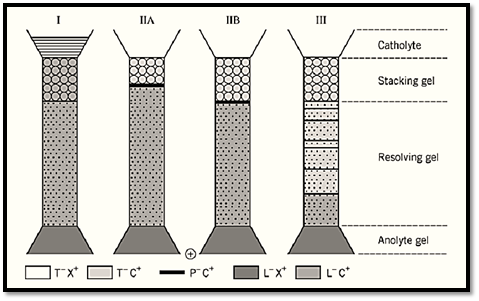
Figure 2. Operation of gel electrophoresis in a discontinuous buffer system. (I) A gel electrophoresis system of four phases is set up: A cathode buffer containing the trailing ion T as a salt with any counterion X plus a tracking dye; a stacking gel of large pore size, “nonrestrictive” to the migration of macromolecular particles P and containing the leading ion L as a salt with counterion C (which is common to both stacking and resolving gels) at a pH lower than that of the resolving gel; a resolving gel at a gel concentration that lowers (“restricts”) the mobility of particle P and at a pH higher than that of the stacking gel; and an anode buffer containing the common counterion C as a salt of any other ion. P is loaded on top of the stacking gel. (II) Upon initiating electrophoresis, a stack of L, P, and T forms by regulation (Fig. 1) in the stacking gel. (III) Upon arrival at the resolving gel, L and T and the tracking dye proceed as a stack at a velocity undiminished by the presence of the gel and augmented by the increased pH. In contrast, the velocity of P is lowered in the gel through “molecular sieving,” so that the mobility of P now is less than that of T. That decrease in mobility is termed “unstacking” and results in the migration of all species of P in decreasing order of their net mobility, ie, in their separation.
Most important, disc electrophoresis is an analytical or preparative separation method that is largely independent of the volume of the original sample; this precludes the necessity for a concentration step prior to electrophoresis. This fact is important for those applications—and they are in the majority—in which the sample is available only in dilute solution. Furthermore, the stacking gel can also assist separation by excluding from the concentrated band (the stack) species of low or high mobility relative to the displacement rate of the moving boundaries associated in the stack (“selective stacking”).
Discontinuous buffer systems are available from a computer program (2) for electrophoresis across the entire pH scale, at either 0 or 25°C, and for both negatively and positively charged analytes.
References
1. L. Ornstein (1964) Ann. NY Acad. Sci. 121, 321–349.
2. A. Chrambach (1985) In The Practice of Quantitative Gel Electrophoresis (V. Neuhoff and A. Maelicke, eds.), VCH, Weinheim, pp. 9–18, 85–110.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)