

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Multiple dilution test
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
20-3-2016
2374
Multiple dilution test
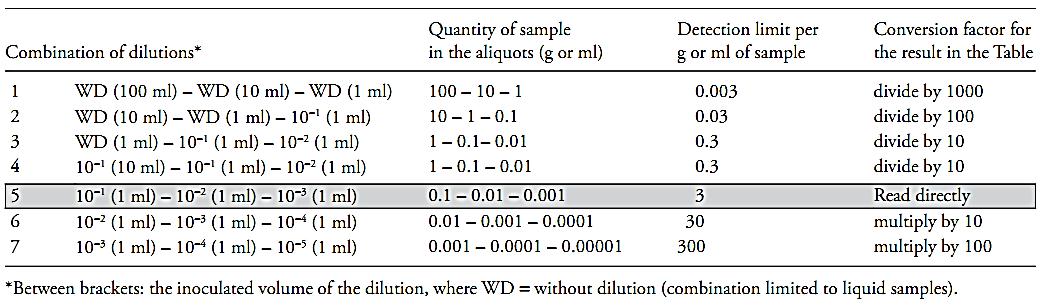
The multiple dilution test is the most versatile format of the MPN technique, since it allows to cover a wide range of microbial concentrations in the sample, by varying the inoculated dilutions. The standard procedure consists in the inoculation of three sequential decimal dilutions of the sample, three aliquots per dilution or, more rarely, five aliquots per dilution and/or five dilutions. The technique, however, also allows for procedures that are not so common, such as the inoculation of a larger number of decimal dilutions, a larger number of aliquots per dilution, or even, non-decimal dilutions. In all these cases, the analytical procedure is the same, but what varies is the way the results are calculated. The standard procedure (three sequential decimal dilutions, three aliquots per dilution) has the advantage that its results can be directly compared against values tabulated in published MPN tables, whereas the non-standard procedures require the use of formulas to make all necessary calculations. The selection of the dilutions will depend on the estimated contamination level of the sample, so as to obtain positive tubes for the smaller dilutions (larger aliquots of the sample) and negative tubes for the greater dilutions (smaller aliquots of the sample). As a rule for guidance, the dilutions recommended for samples with a contamination level in the range of three to 1.000/g or ml, are the 10−1, 10−2 e 10−3 dilutions. If the expected contamination is above this range, higher dilutions should be inoculated. If it is not possible to previously estimate the contamination level of the sample, more than three dilutions (at least five) should be inoculated, beginning with the initial dilution. If the estimated contamination is below this range, greater volumes of undiluted samples (in the case of liquids) or of the first dilution (in the case of sol-ids) may be inoculated, proportionally increasing the volume of culture medium. The proportion between the inoculated volume and the volume of the culture medium recommended by the Compendium is: one part of sample or dilution added to ten parts of broth. A fairly common practice, which maintains this proportion, is to inoculate 10 ml of the liquid samples, without dilution, in 10 ml of double-strength culture medium. This practice is also much used to inoculate the first dilution of solid samples. To guide the selection of dilutions, Table.1 can be consulted. This table shows the quantity of sample present in the aliquots of several combinations of dilutions, along with the detection limit for each combination. Other combinations are possible, particularly in the case of liquid samples, which may be added directly to the broth, such as the decimal combination 100 – 10 –1 ml or the non-decimal combination 500 – 50 –5, for example. In the case of solid samples the options and possibilities are more restricted, since, as already mentioned before, not all products present the homogeneous distribution of microorganisms required for direct inoculation. In the cases in which this does occur, the same combinations as those described for liquid products may be used.
The selection of the culture medium most appropriate for the inoculation of the aliquots will vary according to each test, in function of the target microorganism(s), and is described in the chapters specific to each case.
The verification of the presence of the inoculated target microorganism(s) after the incubation will also vary according to each test and is described in the chapters specific to each case. In most of these tests, this verification includes not only the occurrence of growth, but also the development or not of typical characteristics of the target microorganism(s) in the culture medium used. In addition, most of the tests also require one or two confirmation steps, which are done by transferring the culture obtained in the first inoculated medium to other confirmation media. For example, in one of the test methods for total coliforms, the aliquots are inoculated into Lauryl Sulphate Tryptose Broth (LST), incubated at 35oC/24 h. After the incubation period the tubes are checked for the occurrence or not of growth with gas production. The cultures obtained in the tubes that tested positive for these two characteristics (growth and gas production) are transferred to tubes containing Brilliant Green Bile (BGB) Broth, which is a selective medium for total coliforms. After incubation at 35oC/24 h, the occurrence or not of bacterial growth with gas production is checked again. Only the cultures positive for these two characteristics in BGB, are confirmed as total coliforms. LST broth allows the growth of a series of other gas-producing microorganisms which, in BG broth, are inhibited or differentiated from total coliforms. These cultures are not directly inoculated in BGB because the presence of selective agents may inhibit injured coliforms. The initial LST step ensures the recovery of the injured coliform cells, allowing for later growth under selective conditions.
1 - Material required for the analyses
- Materials for preparing the sample and serial dilutions.
- Culture media recommended for the test to be carried out.
- Laboratory incubator or temperature-controlled water bath set to the temperature specified by the test to be performed.
2 - Procedure
To ensure that all activities will be carried out under aseptic conditions. Properly identify the tubes that will be inoculated by labeling them with the sample code, the dilution, and the standard abbreviation of the culture medium.
a) Preparation of the samples and dilutions.
b) Inoculation. Following the instructions and guide-lines described above, select three or more sequential decimal dilutions of the sample for inoculation. Inoculate three aliquots of each dilution in tubes containing culture broth, specifically selected in accordance with the test to be conducted. For some tests, it is recommended to use a series of five aliquots per dilution . Use a different pipette for each dilution, with a total capacity not greater than 10 ml. The uncertainty of the volume measure-ments should not exceed 5% (ISO 6887-1:1999). Observe carefully whether the tubes being used actually correspond to the sample and the dilution that are being inoculated. Change the position of the tubes as they are being inoculated, to avoid the risk of inoculating the same tube more than one time, or to leave a tube un-inoculated.
c) Incubation. Incubate the tubes under the conditions specified for each test, as described in the spe-cific chapters.
d) Verification of the presence of the target microorganism(s) in the tubes. The definition of the characteristics to be considered as indicative of the presence of the target microorganism(s) in the tubes are defined in the specific chapters. These characteristics may include:
d.1) Growth. Growth is verified by turbidity or cloudiness of the culture medium, provided it is not caused by the sample itself. In the latter case, an alternative procedure may be necessary to confirm growth. One of the most commonly used alternatives is to transfer a loopful of the suspected broth to a new tube containing the same medium and check for turbidity or cloudiness, which would confirm growth in the first tube.
d.2) Gas production. The production of gas can be verified by the formation of bubbles in inverted tubes (Durham tubes), placed inside the broth tubes prior to sterilization. When using this technique, it is important to verify, previously, that no small bubbles were formed in the Durham tubes during storage of the medium in the refrigerator. These bubbles are caused by the air that is dissolved in the liquid and, if present, the tube should be dis-carded and replaced by a new one. Another alternative used to verify the production of gas is to cover the surface of the broth with sealing agar and, after inoculation, observe whether the agar seal has been moved upward as the result of gas formation.
d.3) Acid or base production. The production of acid or base can be verified by the color change of a pH indicator added to the culture broth (bromcresol purple, phenol red and others). Another alternative would be to measure the pH or titrable acidity of the medium after incubation.
d.4) Change of the redox potential. Alteration of the oxide reduction potential is verified by the color change of electron acceptors such as resazurin, methylene blue and triphenyltetrazolium chloride. e) Transfers. Most of the tests that use the MPN technique require the transfer of the culture obtained in the inoculated tubes, to confirm the presence of the target microorganism(s). Only the tubes containing confirmed cultures are considered as positive to calculate the MPN.
Table.1 Guide for the use of the MPN tables.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
International Organization for Standardization (1999) ISO 6887-1:1999. Microbiology of food and animal feeding stuffs – Prepa-ration of test samples, initial suspension and decimal dilutions for microbiological examination – Part 1: General rules for the prepara-tion of the initial suspension and decimal dilutions. Geneva, ISO.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















