
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
ABSORPTION AND EMISSION PROCESSES
المؤلف:
Mark Csele
المصدر:
FUNDAMENTALS OF LIGHT SOURCES AND LASERS
الجزء والصفحة:
p10
7-3-2016
3196
ABSORPTION AND EMISSION PROCESSES
Light is a product of quantum processes occurring when an electron in an atom is excited to a high-energy state and later loses that energy. Imagine an atom into which energy is injected (the method may be direct electrical excitation or simply thermal energy provided by raising the temperature of the atom). The electron acquires the energy and in doing so enters an excited state. From that excited state the electron can lose energy and fall to a lower-energy state, but energy must be conserved during this process, so the difference in energy between the initial high-energy state and the final low-energy state cannot be destroyed; it appears either as a photon of emitted light or as energy transferred to another state or atom. This is simply the principle of conservation of energy. The fact that atoms and molecules have such energy levels and transitions can occur between these
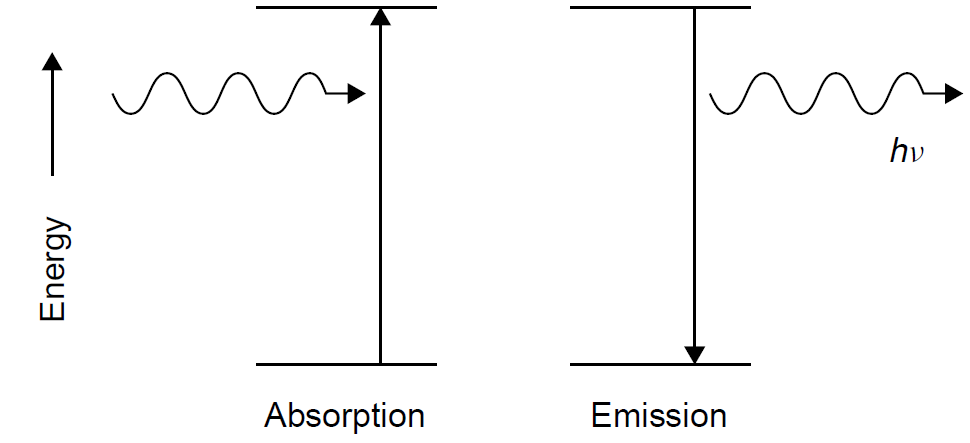
Figure 1.1. Absorption and emission of energy.
An atom at a low energy state can absorb energy and in so doing will be elevated to a higher-energy state. The energy absorbed can be in almost any form, including electrical, thermal, optical, chemical, or nuclear. The difference in energy between the original (lower) energy state and the final (upper) energy state will be exactly the energy that was absorbed by the atom. This process of absorption serves to excite atoms into high-energy states. Regardless of the excitation method, an atom in a high energy state will certainly fall to a lower-energy state since nature always favors a lower-energy state (i.e., the law of entropy from thermodynamics). In jumping from a high- to a low-energy state, photons will be produced, with the photon energy being the difference in energy between the two atomic energy states. This is the process of emission (Figure 1.1). By knowing the energies of the two atomic states involved, we may predict the wavelength of the emitted photon using Planck’s relationship according to
Ephoton = Einitial - Efinal = hvphoton
Examining an incandescent light bulb, we have a thin filament of tungsten metal glowing white-hot and emitting light. The energy is supplied in the form of an electrical current passing through the filament. In doing so, the filament is raised in temperature to 3000 K or more (the atmosphere inside a light bulb must be void of oxygen or the filament would quickly burn). This thermal energy excites tungsten atoms in the filament to high energy levels, but nature favors low-energy states, so the atoms will not stay in these high-energy states indefinitely. Soon, excited atoms will lose their energy by emitting light and falling to lower-energy states. The amount of energy lost by the atom appears as light. The low energy atoms are now free to absorb thermal energy and to begin the process again. As long as energy is supplied to the system, tungsten atoms will continue this process of absorption of energy (in this case, thermal energy) and emission of light. Not all energy emitted is in the form of visible light. The largest portion of the electrical energy injected into the system appears as heat and infrared light. Various forms of light use various means of excitation. In a neon sign, for example, an electrical discharge (usually at high voltages) pumps atoms to high energy states. Gases usually have well-defined energy levels (as for the example of hydrogen in Figure 1.2), so their emissions often appear as line spectra: a series of discrete emissions (lines) of well-defined wavelengths. In the case of a fluorescent tube, a gas discharge emits radiation that excites phosphor on the wall of the tube. The phosphor then emits visible light from the tube. We examine, as they also provide insight into quantization. Other common forms of light include chemical glow sticks (the type you bend to break an inner tube to start them glowing). A reaction between two chemicals in the tube (previously separated but now mixed) excites atoms to high energy states. In this chapter we examine primarily thermal light in which excitation is brought about solely by temperature. To reiterate, all atoms and molecules have energy levels such as those depicted in Figure 1.2, which shows the levels for a hydrogen atom. Light is produced when an atom at a higher energy level loses energy and falls to a lower level in what is called a radiative transition (so called because radiation is emitted in the transition). Two such transitions are shown on the figure, one emitting red light and the other emitting blue light. Energy in such a transition must be conserved, so the difference in energy between the upper and lower levels is released as a photon of light. The larger the transition, the more energy the photon will have (e.g., a blue photon has more energy than a red photon). As well as emitting photons, there are also non-radiative transitions in which energy is lost without emission of radiation (e.g., as heat). These do not contribute to radiation emission, so are omitted from this discussion. Evident in Figure 1.2 is a level labeled the ground state of the atom. This is the lowest energy state that an atom or molecule can have. All atoms and molecules
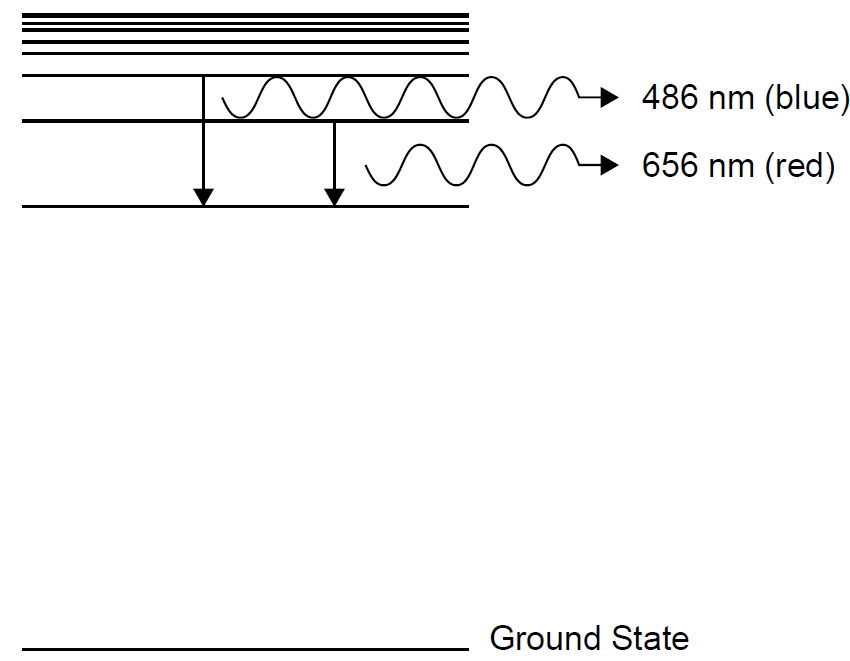
Figure 1.2. Simplified hydrogen energy levels.
assume this state at absolute zero (0 K), but frequently, the addition of energy (from electrical or thermal sources) causes the energy of the atom to rise to an upper level. The radiative transitions in Figure 1.2 are between two upper energy levels in the atom but could also be between an upper energy level and the ground state. In this case the transition would result in a higher energy photon than those shown in the figure, and ultraviolet radiation would result. Knowing where the energy levels are in a particular atom (and these are well known for essentially all atomic and molecular species), the problem now is to determine how many atoms are at a particular energy state. To do this, we use a thermodynamic concept called the Boltzmann energy distribution.
 الاكثر قراءة في الضوء
الاكثر قراءة في الضوء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















