


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2025-04-15
Date: 2025-05-01
Date: 2025-05-04
|
The Pathological Immune Response
An immune response can also cause disease. Such responses can be classified into the following types: Type I: allergic IgE-dependent diseases; Type II: antibody-dependent responses to cell membranes, blood group antigens or other auto-antigens; Type III: immune complex-initiated diseases whereby surplus antigen-antibody complexes are deposited on basement membranes, resulting in development of chronic disease via complement activation and inflammatory reactions; Type IV: cellular immunopathology resulting from excessive T-cell responses against infections that otherwise exhibit low cytopathogenicity, or against allogenic organ transplants.
Type I: IgE-Triggered Anaphylaxis
This type of immediate hypersensitivity reaction occurs within minutes in allergically sensitized individuals. Although serum IgE has a short half-life (one to two days), IgE antibodies bound to the Fce receptor on basophils and mast cells have a half-life of several months and when bound by the specific allergen mediate cellular degranulation and the release of biogenic amines (e.g., histamine, serotonin). These mediators can influence the smooth musculature, and mainly result in the constriction of the pulmonary- and broncho-postcapillary venules, together with arteriole dilation. The local manifestations of IgE-triggered anaphylaxis include whealing of the skin (urticaria), diarrhea for food allergies, rhinitis or asthma for pollen allergies, or a generalized anaphylactic shock. IgE reactions are usually measured in vitro using RIA (radioimmunoassay), RIST (radioimmunosorbent test) or RAST (radioallergosorbent test) Frequent causal agents of IgE allergies in humans include pollen, animal hair, house dust (mites), insect bites and stings, penicillin, and foods. Examples of allergic diseases include local allergic rhinitis and conjunctivitis, allergic bronchial asthma, systemic anaphylactic shock, insect toxin allergies, house dust (mite) and food allergies, urticaria, and angioedemas.
Degranulation of mast cells and basophils can be induced by factors other than the cross-linking of specific IgE antibodies. Such factors include the complement factors C3a and C5a, and pharmacological inducers (“pseudo-allergy!”).
Atopic patients suffer severely from allergies. Atopia is genetically conditioned, with a child exhibiting a 50% risk of developing atopy if both parents are allergic, or a 30% risk if only one parent is allergic. The incidence level of atopy within the general population is roughly 10-15%. Atopia correlates with high levels of IgE production, and desensitization refers to attempts to change a TH2 (IgE-producing) response into a TH1 (IgG-favoring) response by means of repeated inoculations or oral doses of allergens. It is likely that increased production of IgG—as opposed to IgE—anti- bodies plays a major role in the success of desensitization. IgE no doubt has an important biological function, probably against ectoparasites, with allergic reactions representing nothing more than an unfortunate side effect of this biological system. Little research has been performed on the nature of the protective function of IgE during parasitic infections (or on the role of eosinophils). However, we do know that mediators released by IgE-triggering of mast cells and basophils cause the smooth intestinal musculature to contract, and in this way facilitate the elimination of intestinal parasites.
Type II: Cytotoxic Humoral Immune Responses
These are pathological immune responses induced by the binding of IgM or IgG antibodies to antigens present on a cell surface (including viral products or haptens), or within tissue components. The mediators responsible for such tissue damage are usually components of the complement system, or granulocytic digestive enzymes. The most important diseases resulting from cytotoxic humoral immune responses are listed in Table 1.
Table 1 Examples of Antibody–Related Type II Immunopathologies
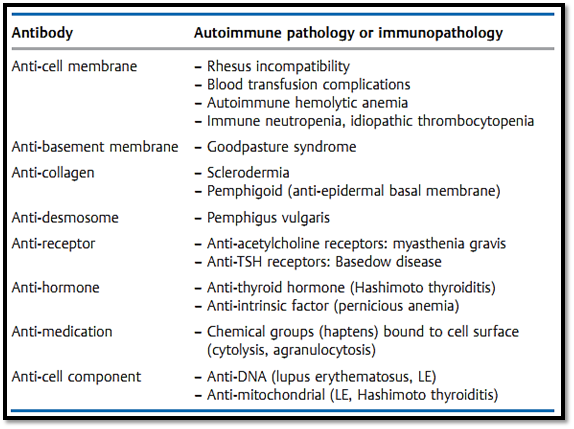
Autoantibody Responses
Some clinically important autoantibodies are directed against hormone receptors, for example thyrotoxicosis in Basedow's disease is caused by autoantibodies that stimulate the TSH receptor, and myasthenia gravis is caused by blockage of the acetylcholine receptor by specific autoantibodies. Other antibody-induced diseases mediated by antibodies, directed against hormones and other cellular self antigens, include Hashimoto thyroiditis (induced by anti-thyroglobulin and anti-mitochondrial autoantibodies), pernicious anemia (anti-intrinsic factor), pemphigus vulgaris (anti-desmosome) Guillain-Barre syndrome (ascending paralysis caused by specific myelin autoantibodies), and scleroderma (involving anti-collagen antibodies). Other immunopathologies involving autoantibodies include transplant rejection as a result of endothelial damage (especially in xenogeneic transplants), and tumor rejection caused by antibodies against tumor-associated antigens present on neoplastic cells (especially relevant for lymphohematopoietic tumors). However, in general the detection of autoantibodies does not necessarily correlate with evidence of pathological changes or processes. In fact, our detection methods often measure low-avidity autoantibodies that may have no direct disease-causing effects.
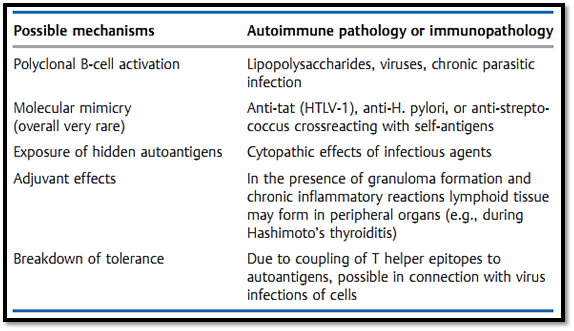
Exactly how autoantibody responses are induced remains to be clarified. As explained earlier (in the discussion of immunological tolerance) such IgG responses cannot be induced without T help. Thus, intensive research is currently focused on those mechanisms by which T cell help for autoreactive B cells is regulated; Table 2 sums up some of the possible mechanisms.
Table 2 Mechanisms Of Autoantibody Induction
Anti-blood Group Antibody Reactions
ABO system. These B-cell epitopes consist of sugar groups present in the membranes of red blood cells. The four classic blood groups are determined by one gene with three alleles. This gene controls glycosylation. The O allele codes only for a basic cell surface structure (H substance) with the terminal sugars galactose and fucose. The A allele adds N-acetylgalactosamine to this basic structure, the B allele adds galactose. This results in epitopes, which are also seen frequently in nature largely as components of intestinal bacteria. Individuals who carry the A allele are tolerant to the A-coded epitope, whilst individuals with the B allele are tolerant to the B epitope. Individuals who carry both of these alleles (genotype AB) are tolerant to both epitopes, whereas persons who are homozygotes for the O allele are not tolerant to either A or B. Following birth, the intestinal tract is colonized by bacteria containing large numbers of epitopes similar to the A and B epitopes. During the first months of life, people with blood group O (homozygous for the O allele) produce both anti-A and anti-B antibodies, people with blood group A (genotype AO or AA) produce only anti-B antibodies, people with blood group B (genotype BO or BB) produce only anti-A antibodies, and people with blood group AB produce neither anti-A or anti-B antibodies.
These so-called “natural” antibodies (meaning these antibodies are produced without a recognizable immunization process) are of the IgM class; there is usually no switch to IgG, probably resulting from a lack of necessary helper T-cell epitopes. The presence of the blood group antibodies makes blood transfusions between non-matched individuals extremely risky, necessitating that the blood group of both the donor and recipient is determined before the blood transfusion takes place. Nevertheless, the antibodies in the donor blood are not so important because they are diluted. The O genotype is therefore a universal donor. Note that IgM antibodies to blood groups present no danger to the fetus since they cannot pass through the placental barrier.
Rhesus factor. This system is also based on genetically determined antigens present on red blood cells, although as a general rule there is no production of “natural” antibodies against these. IgM and IgG antibodies are not induced unless an immunization (resulting from blood transfusion or pregnancy) takes place. During the birth process, small amounts of the child's blood often enter the mother's bloodstream. Should the child's blood cells have paternal antigens, which are lacking in the mother's blood, his or her blood will effectively 'immunize' the mother. Should IgG antibodies develop they will represent a potential risk during subsequent pregnancies should the fetus once again present the same antigen. The resulting clinical picture is known as morbus hemolyticus neonatorum or erythroblastosis fetalis (“immune hydrops fetalis”).
Once immunization has occurred, thus endangering future pregnancies, genetically at risk children can still be saved by means of cesarean section and exchange blood transfusions. Should the risk of rhesus immunization be recognized at the end of the first pregnancy, immunization of the mother can be prevented by means of a passive infusion of antibodies against the child's antigen, immediately following the birth. This specific immunosuppressive procedure is an empirical application of immunological knowledge, although the precise mechanism involved is not yet been completely understood.
Other blood group systems. There are other additional blood group systems against which antibodies may be produced, and which can present a risk during transfusions. Thus, the crossmatch test represents an important measure in the avoidance of transfusion problems. Immediately prior to a planned transfusion, serum from the prospective recipient is mixed with erythrocytes from the prospective donor, and serum from the prospective donor is mixed with erythrocytes from the prospective recipient. To ensure no reaction following transfusion, there should be no agglutination present in either mixture. Some potentially dangerous serum antibodies may bind to the erythrocytes causing opsonization, but not necessarily inducing agglutination. To check for the presence of such antibodies, anti-human immunoglobulin serum is added and should it crosslink such antibodies agglutination will result.
Type III: Diseases Caused by Immune Complexes
Pathologies initiated by immune complexes result from the deposition of small, soluble, antigen-antibody complexes within tissues. The main hallmark of such reactions is inflammation with the involvement of complement. Normally, large antigen-antibody complexes (that is, those produced in equivalence) are readily removed by the phagocytes of the reticuloendothelial system. Occasionally, however—especially in the presence of persistent bacterial, viral, or environmental, antigens (e.g., fungal spores, vegetable or animal materials), or during autoimmune diseases directed against autoantigens (e.g., DNA, hormones, collagen, IgG) where autoantibodies to the body's own antigens are produced continuously—deposition of antigen-antibody complexes may become widespread often being present on active secretory membranes and within smaller vessels. Such processes are mainly observed within infected organs, but can also occur within kidneys, joints, arteries, skin and lung, or within the brain's plexus choroideus. The resulting inflammation causes local tissue damage. Most importantly, activation of complement by such complexes results in production of inflammatory C components (C3a and C5a). Some of these anaphylatoxins cause the release of vasoactive amines which increase vascular permeability (see also p. 103f.). Additional chemotactic activities attracts granulocytes which attempt to phagocytize the complexes. When these phagocytes die, their lysosomal hydrolytic enzymes are released and cause further tissue damage. This process can result in long-term chronic inflammatory reactions.
There are two basic patterns of immune complex pathogenesis:
-Immune complexes in the presence of antigen excess. The acute form of this disease results in serum sickness, the chronic form leads to the development of arthritis or glomerulonephritis. Serum sickness often resulted from serum therapy used during the pre-antibiotic era, but now only occurs rarely. Inoculation with equine antibodies directed against human pathogens, or bacterial toxins, often induced the production of host (human) antibodies against the equine serum. Because relatively large amounts of equine serum were administered for such therapeutic purposes, such therapy would result in the induction of antigen-antibody complexes—some of which were formed in the presence of antigen excess—and occasionally induced a state of shock.
-Immune complexes in the presence of antibody excess. The so-called Arthus reaction is observed when an individual is exposed to repeated small doses of an antigen over a long period of time, resulting in the induction of complexes and an antibody excess. Further exposure to the antigen, particularly dermal exposure, induces a typical reaction of edema and erythema which peaks after three to eight hours and disappears within 48 hours, but which sometimes leads to necrosis. Arthus-type reactions often represent occupational diseases in people exposed to repeated doses of environmental antigens: farmer's lung (thermophilic Actinomyces in moldy hay), pigeon breeder's lung (protein in the dust of dried feces of birds), cheese worker's lung (spores of Penicillium casei), furrier's lung (proteins from pelt hairs), malt-worker's lung (spores of Aspergillus clavatus and A. fumigatus).
Type IV: Hypersensitivity or Delayed Type,
Cell-Mediated Hypersensitivity
Intracutaneous injection of a soluble antigen derived from an infectious pathogen induces a delayed dermal thickening reaction in those people who have suffered a previous infection. This delayed skin reaction can serve as a test to confirm immunity against intracellular bacteria or parasites.
For most cases, the time between administration of the antigen and the swelling reaction is 48-72 hours—as described above for cellular delayed type hypersensitivity (DTH) reactions in the skin. As observed for antibody- dependent hyper-reactions of types I-III, the type IV response is pathogenic and differs from protective immune responses only in terms of the extent and consequences of the tissue damage, but not in terms of the mechanism of action. The balance between autoimmune disease and type IV immunopathology in such cases is readily illustrated by type IV reactions (e.g., aggressive hepatitis in humans or lymphocytic choriomeningitis in mice). Should the causal infectious pathogen be known, the response is termed a type IV reaction, if the causal agent is unknown (or not yet determined) the same condition may be termed “autoimmune disease.” The reader is referred to the many examples of type IV responses already discussed within various chapters (DTH [p. 99], immune protection and immunopathology, transplantation immunology [see below], and autoimmunity.
Autoimmune T cells are usually directed against autoantigens that would otherwise be ignored (since they are only expressed in the extralymphatic periphery). Autoaggressive CD4+ T cells apparently respond against myelin basic protein in multiple sclerosis, against collagen determinants in polyarthritis, and against islet cell components in diabetes.
References
Zinkernagel, R. M. (2005). Medical Microbiology. Thieme.

|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
شعبة مدارس الكفيل: مخيَّم بنات العقيدة يعزِّز القيم الدينية وينمِّي مهارات اتخاذ القرار لدى المتطوِّعات
|
|
|