


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيويةEpigenetic Effects Can Be Inherited
KEY CONCEPTS
- Epigenetic effects can result from modification of a nucleic acid after it has been synthesized without changing the DNA sequence or by the perpetuation of protein structures.
- Epigenetic effects may be inherited through generations.
- Aberrant epigenetic inheritance may be preventable.
Epigenetic inheritance describes the ability of different states, which may have different phenotypic consequences, to be inherited without any change in the sequence of DNA. How can this occur? Epigenetic mechanisms can be divided into two general classes:
- DNA may be modified by the covalent attachment of a moiety that is then perpetuated. Two alleles with the same sequence may have different states of methylation that confer different properties.
- A self-perpetuating protein state may be established. This might involve assembly of a protein complex, modification of specific protein(s), or establishment of an alternative protein conformation.
Methylation establishes epigenetic inheritance so long as the maintenance methyltransferase acts constitutively to restore the methylated state after each cycle of replication. A state of methylation can be perpetuated through an indefinite series of somatic mitoses. This is probably the “default” situation. Methylation can also be perpetuated through meiosis. For example, in the fungus Ascobolus epigenetic effects can be transmitted through both mitosis and meiosis by maintaining the state of methylation. In mammalian cells, epigenetic marks are first erased in primordial germ cells and then reestablished in new patterns by resetting the state of methylation differently in male and female meioses during gametogenesis.
Situations in which epigenetic effects appear to be maintained by means of protein states are less well understood in molecular terms. PEV shows that constitutive heterochromatin may extend for a variable distance, and the structure is then perpetuated through somatic divisions. There is no methylation of DNA in Saccharomyces and a vanishingly small amount in Drosophila, and as a result the inheritance of epigenetic states of PEV or telomeric silencing in these organisms is likely to be due to the perpetuation of protein structures.
FIGURE 1 considers two extreme possibilities for the fate of a protein complex at replication:
- A complex could perpetuate itself if it splits symmetrically, so that half complexes associate with each daughter duplex. If the half complexes have the capacity to nucleate formation of full complexes, the original state will be restored. This is basically analogous to the maintenance of methylation. The problem with this model is that there is no evident reason why protein complexes should behave in this way.
- A complex could be maintained as a unit and segregate to one of the two daughter duplexes. The problem with this model is that it requires a new complex to be assembled de novo on the other daughter duplex, and it is not evident why this should happen.
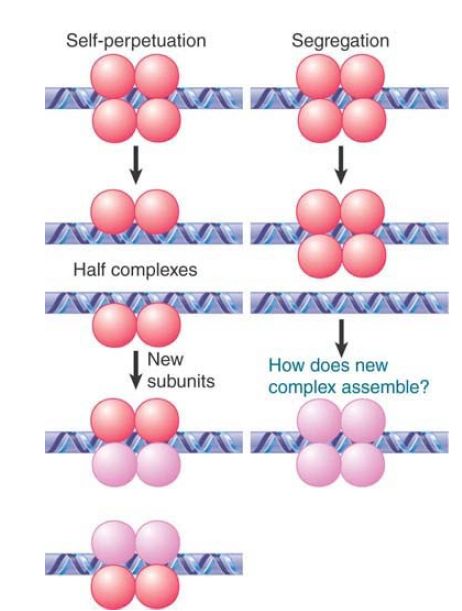
FIGURE 1. What happens to protein complexes on chromatin during replication?
Consider now the need to perpetuate a heterochromatic structure consisting of protein complexes. As described earlier, random distribution of proteins to each daughter duplex at replication can result in restoration of the heterochromatic state if the protein has a self-assembling property that causes new subunits to associate with it .
In some cases, it may be the state of protein modification, rather than the presence of the protein per se, that is responsible for an epigenetic effect. A general correlation exists between the activity of chromatin and the state of acetylation of the histones, in particular the acetylation of the N-terminal tails of histones H3 and H4. Activation of transcription is associated with acetylation in the vicinity of the promoter, and repression of transcription is associated with deacetylation . The most dramatic correlation is that the inactive X chromosome in mammalian female cells is underacetylated.
The inactivity of constitutive heterochromatin may require that the histones are not acetylated. If a histone acetyltransferase is tethered to a region of telomeric heterochromatin in yeast, silenced genes become active. When yeast is exposed to trichostatin (an inhibitor of deacetylation), centromeric heterochromatin becomes acetylated, and silenced genes in centromeric regions may become active. The effect may persist even after trichostatin has been removed. In fact, it may be perpetuated through mitosis and meiosis. This suggests that an epigenetic effect has been created by changing the state of histone acetylation.
How might the state of acetylation be perpetuated? Suppose that the H32 –H42 tetramer is distributed at random to the two daughter duplexes. This creates the situation shown in FIGURE 2 , in which each daughter duplex contains some histone octamers that are acetylated on the H3 and H4 tails, whereas others are unacetylated. To account for the epigenetic effect, we could suppose that the presence of some acetylated histone octamers provides a signal that causes the unacetylated octamers to be acetylated.
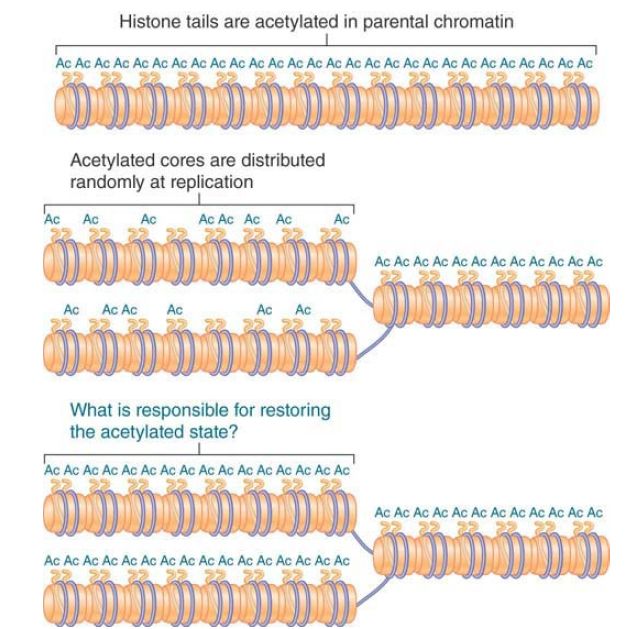
FIGURE 1. Acetylated histones are conserved and distributed at random to the daughter chromatin fibers at replication. Each daughter fiber has a mixture of old (acetylated) cores and new (unacetylated) histones.
It is not yet fully understood how epigenetic changes are inherited mitotically in somatic cells, but it is clear that this occurs. Surprisingly, several lines of evidence indicate that epigenetic effects may also be transmitted across generations in a process referred to as transgenerational epigenetics. Evidence that DNA methylation is a central coordinator that secures stable transgenerational inheritance in plants comes from studies of an Arabidopsis thaliana mutant deficient in maintaining DNA methylation. The loss of DNA methylation triggers genome-wide activation of alternative epigenetic mechanisms such as RNAdirected DNA methylation, DNA demethylase inhibition, and retargeting of histone H3K9 methylation. In the absence of maintenance methylation, new and aberrant patterns of epigenetic marks accumulate over several generations, leaving these plants dwarfed and sterile. As a result—at least in plants—the case is strong that intact maintenance methylation plays a major role in transgenerational epigenetics.
In mammals, support for transgenerational epigenetics is less strong, but several lines of evidence indicate that this process occurs in mammals as well. Metastable epialleles are dependent on the epigenetic state for their transcription. This state can vary not only between cells but also between tissues. Although the epigenetic state of the genome undergoes reprogramming in the parental genomes and during early embryogenesis, some loci may transmit the epigenetic state through the gametes to the next generation (transgenerational epigenetics). For example, in mice there is a dominant mutation of the agouti locus (a coat color gene) known as agouti viable yellow, which is caused by the insertion of a retrotransposon upstream of the agouti coding region. This allele shows variegation, resulting in coat colors ranging from solidyellow, to mottled, to compl etely agouti (dark). It has been observed that agouti females are more likely to produce agouti offspring and yellow females are more likely to produce yellow offspring—in other words, the variable level of expression of agouti in the mother appears to be transmitted to the offspring (while the color of the father is irrelevant). It turns out that DNA methylation of the inserted retrotransposon determines the coat color of the agouti mice, indicating transgenerational conservation of expression levels due to incomplete erasure of the epigenetic mark between generations.
Metastable alleles may also play a role in transgenerational epigenetic inheritance in humans, as suggested by the high degree of copy-number variation within monozygotic twins. Moreover, in some cases of Prader–Willi syndrome no mutation is apparent, but there is an epimutation involving aberrant DNA methylation. The cause for the epimutation may be due to an allele that has passed through the male germline without erasure of the silent epigenetic state established in the grandmother. Thus, the evidence for transgenerational epigenetic inheritance is emerging not only in plants and mammals but also as a potential cause for gene control or diseases due to aberrant epigenetic control of transcription inhumans.
As an interesting and important extension of this concept, a number of human diseases may have an etiological basis in transgenerational epigenetic inheritance that may be preventable. For example, in utero exposure can occur from certain diets that have epigenetic-modifying potential through their bioactive compounds, such as maternal diets lacking methyl donors (e.g.,folate or choline) that result in lifelong undermethylation of certain regions in the offspring. This could lead to reprogramming of primary epigenetic profiles such as DNA methylation and histone modifications in the fetal genome that could impact disease risk later in life.

|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
لأعضاء مدوّنة الكفيل السيد الصافي يؤكّد على تفعيل القصة والرواية المجسّدة للمبادئ الإسلامية والموجدة لحلول المشاكل المجتمعية
|
|
|
|
قسم الشؤون الفكرية يناقش سبل تعزيز التعاون المشترك مع المؤسّسات الأكاديمية في نيجيريا
|
|
|
|
ضمن برنامج عُرفاء المنصّة قسم التطوير يقيم ورشة في (فنّ الٕالقاء) لمنتسبي العتبة العباسية
|
|
|
|
وفد نيجيري يُشيد بمشروع المجمع العلمي لحفظ القرآن الكريم
|