


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 12-5-2016
Date: 19-5-2021
Date: 1-5-2016
|
Controlling the Direction of Mismatch Repair
KEY CONCEPTS
- The prokaryotic mut genes encode mismatch repair proteins.
- Bias exists in the selection of which strand to replace at mismatches.
- The strand lacking methylation at a hemimethylated  is usually replaced.
is usually replaced.
- The mismatch repair system is used to remove errors in a newly synthesized strand of DNA. At G-T and C-T mismatches, the thymine is preferentially removed.
- Eukaryotic MutS/L systems repair mismatches and insertion/deletion loops.
Genes whose products are involved in controlling the fidelity of DNA synthesis during either replication or repair may be identified by mutations that have a mutator phenotype. A mutator mutant has an increased frequency of spontaneous mutation. If identified originally by the mutator phenotype, a prokaryotic gene is described as mut; often, though, a mut gene is later found to be equivalent with a known replication or repair activity.
Many mut genes turn out to be components of mismatch repair systems. Failure to remove a damaged or mispaired base before replication allows it to induce a mutation. Functions in this group include the Dam methylase that identifies the target for repair and enzymes that participate directly or indirectly in the removal of particular types of damage (MutH, -S, -L, and -Y).
When a helix-distorting bulky lesion is removed from DNA, the wildtype sequence is restored. In most cases, the distortion is due to the creation of a base that is not naturally found in DNA and that is therefore recognized and removed by the repair system.
A problem arises if the target for repair is a mispaired partnership of (normal) bases created when one was mutated or misinserted during replication. The repair system has no intrinsic means of knowing which is the wild-type base and which is the mutant. All it sees are two improperly paired bases, either of which can provide the target for excision repair.
If the mutated base is excised, the wild-type sequence is restored. If it happens to be the original (wild-type) base that is excised, though, the new (mutant) sequence becomes fixed. Often, however, the direction of excision repair is not random, but instead is biased in a way that is likely to lead to restoration of the wildtype sequence.
Some precautions are taken to direct repair in the right direction. For example, for cases such as the spontaneous deamination of 5-methylcytosine to thymine, a special system restores the proper sequence. This deamination event generates a G-T pair, and the system that acts on such pairs has a bias to correct them to G-C pairs (rather than to A-T pairs). The system that undertakes this reaction includes the MutL and MutS products that remove thymine from both G-T and C-T mismatches.
The MutT, -M, -Y system handles the consequences of oxidative damage. A major type of chemical damage is caused by oxidation of guanine to form 8-oxo-G, which can occur in GTP or when guanine is present in DNA. FIGURE 1. shows that the system operates at three levels. MutT hydrolyzes the damaged precursor 8-oxo-dGTP, which prevents it from being incorporated into DNA.
When guanine is oxidized in DNA its partner is cytosine, and MutM preferentially removes the 8-oxo-G from 8-oxo-G-C pairs. However, oxidized guanine mispairs with adenine, and so if 8-oxo-G persists in DNA and is replicated, it generates an 8-oxo-G-A pair.
MutY removes adenine from these pairs. MutM and MutY are glycosylases that directly remove a base from DNA. This creates an apurinic site that is recognized by an endonuclease whose action triggers the involvement of the excision repair system.

FIGURE 1. Preferential removal of bases in pairs that have oxidized guanine is designed to minimize mutations.
When mismatch errors occur during replication in E. coli, it is possible to distinguish the original strand of DNA. Immediately after replication of methylated DNA, only the original parental strand carries methyl groups. In the period during which the newly synthesized strand awaits the introduction of methyl groups, the two strands can be distinguished. This provides the basis for a system to correct replication errors. The dam gene encodes a methyltransferase whose target is the adenine in the sequence CTAG. The hemimethylated state is used to distinguish replicated origins from nonreplicated origins. The same target sites are used by a replication-related mismatch repair system.
FIGURE 2 shows that DNA containing mismatched base pairs is repaired by preferentially excising the strand that lacks the methylation. The excision is quite extensive; mismatches can be repaired preferentially for as much as 1 kb around a GATC site. The result is that the newly synthesized strand is corrected to the sequence of the parental strand.
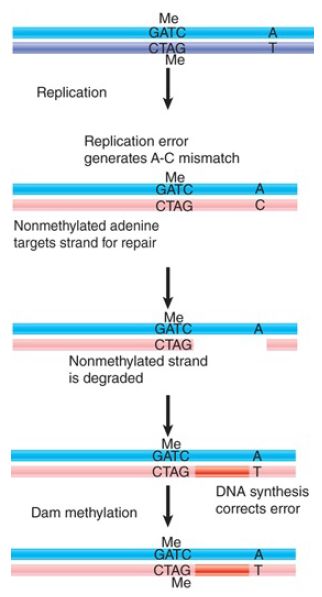
FIGURE 2. GATC sequences are targets for Dam methylase after replication. During the period before this methylation occurs, the nonmethylated strand is the target for repair of mismatched bases.
E. coli dam− mutants show an increased rate of spontaneous mutation. This repair system therefore helps reduce the number of mutations caused by errors in replication. It consists of several proteins coded by mut genes. MutS binds to the mismatch and is joined by MutL. MutS can use two DNA-binding sites, as illustrated in FIGURE 3. The first specifically recognizes mismatches. The second is not specific for sequence or structure and is used to translocate along DNA until a GATC sequence is encountered. Hydrolysis of ATP is used to drive the translocation. MutS is bound to both the mismatch site and DNA as it translocates, and as a result it creates a loop in the DNA.
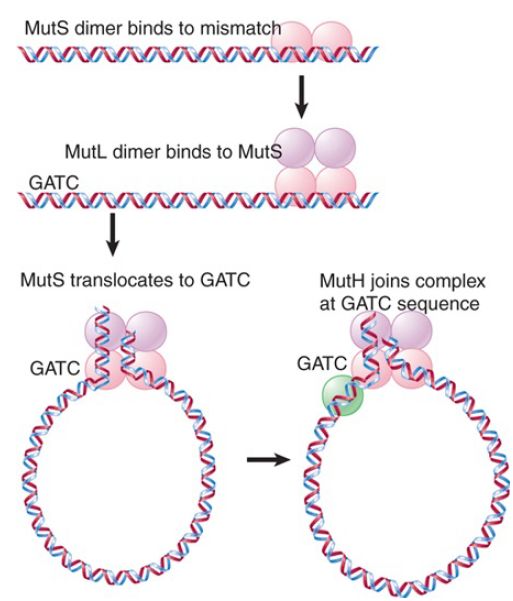
FIGURE 3. MutS recognizes a mismatch and translocates to a GATC site. MutH cleaves the unmethylated strand at the GATC. Endonucleases degrade the strand from the GATC to the mismatch site.
Recognition of the GATC sequence causes the MutH endonuclease to bind to MutS/L. The endonuclease then cleaves the unmethylated strand. This strand is then excised from the GATC
site to the mismatch site. The excision can occur in either the 5′ →3′ direction (using RecJ or exonuclease VII) or in the 3′ → 5′ direction (using exonuclease I) and is assisted by the helicase UvrD. A new DNA strand is then synthesized by DNA polymerase III.
Eukaryotic cells have systems homologous to the E. coli mut system. Msh2 (“MutS homolog 2”) provides a scaffold for the apparatus that recognizes mismatches. Msh3 and Msh6 provide specificity factors. In addition to repairing single-base mismatches, they are responsible for repairing mismatches that arise as the result of replication slippage. The hMutSβ complex, a Msh2–Msh3 dimer, binds mismatched insertion/deletion loops, whereas the Msh2–Msh6 (hMutSα) complex binds to single-base mismatches.
Other proteins, including the MutL homolog hMutLα (a dimer of Mlh1 and Pms2), are required for the repair process itself. Surprisingly, even though multicellular eukaryotes possess DNA methylation that must be restored after replication just as in prokaryotes, eukaryotic mismatch repair systems do not use DNA methylation to select the daughter strand for repair. Eukaryotes recognize the daughter strand during mismatch repair via direct interactions with the replication machinery and preferentially recognizing strands containing nicks as daughter stands. Nicks between Okazaki fragments can serve this purpose on the lagging strand, and hMutLα itself creates DNA ends to use for repair.
hMutLα DNA nicking is activated by the replication factor PCNA, which is oriented so as to direct the activity of the repair endonuclease to the nascent daughter strand.
The eukaryotic hMutS/L system is also particularly important for repairing errors caused by replication slippage. In a region such as a microsatellite, where a very short sequence is repeated a number of times, realignment between the newly synthesized daughter strand and its template can lead to a “stuttering” in which the DNA polymerase slips backward and synthesizes extra repeating units or slips forward and skips repeats. The mismatched repeats are extruded as single-stranded insertion-deletion loops (“indels”) from the double helix, which are repaired by homologs of the hMutS/L system, as shown in FIGURE 4. Failure to repair
insertion-deletion loops leads to repeat contraction or expansion. A number of human diseases, including Huntington’s and Fragile X syndrome, are caused by repeat expansions.

FIGURE 4. The MutS/L system initiates repair of mismatches produced by replication slippage.
The importance of the hMutS/L system for mismatch repair is indicated by the high rate at which it is found to be defective in human cancers. Loss of this system leads to an increased mutation rate, and germline mutations in hMutS/L components can lead to Lynch syndrome. These patients have increased risk of colorectal and other cancers (this syndrome has also been called hereditary nonpolyposis colorectal cancer, or HNPCC). A characteristic feature of Lynch syndrome is microsatellite instability, in which the lengths (numbers of repeats) of microsatellite sequences change rapidly in the tumor cells due to the loss of the mismatch repair system to correct replication slippage in these sequences. This instability has been used diagnostically to identify Lynch syndrome, but this method has been mostly replaced by immunohistochemistry (IHC) to detect loss of MMR factors in tumor tissue.

|
|
|
|
كيف تعزز نمو الشعر الصحي؟
|
|
|
|
|
|
|
10 فحوصات مهمة يجب القيام بها لسيارتك قبل الصيف
|
|
|
|
|
|
العتبة العباسية تختتم فعاليات حفل التكليف الشرعي في قضاء عين التمر بكربلاء
|
|
|
|
طالبات مدارس عين التمر يرددن نشيد التكليف الشرعي
|
|
|
|
الطالبات المشاركات في حفل التكليف الشرعي يقدمن الشكر للعتبة العباسية
|
|
|
|
حفل التكليف الشرعي للطالبات يشهد عرض فيلم تعريفي بمشروع (الورود الفاطمية)
|