


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 28-4-2016
Date: 11-5-2016
Date: 15-4-2021
|
Insulators Define Transcriptionally Independent Domains
KEY CONCEPTS
-Mammalian chromosomes are organized as strings of topologically associated domains (TADs) that average about 1 megabase (Mb) in size.
-TADs or TAD-like structures have been found in most eukaryotes.
-Loci within a TAD interact frequently with each other, but less frequently with loci in an adjacent TAD.
-TAD organization is fairly stable between cells, but interactions within TADs are highly dynamic.
-Boundary regions between TADs contain insulator elements that are able to block passage of any activating or inactivating effects from enhancers, silencers, and other control elements.
-Insulators can provide barriers against the spread of heterochromatin.
-Insulators are specialized chromatin structures that typically contain hypersensitive sites.
-Different insulators are bound by different factors and may use alternative mechanisms for enhancer blocking and/or heterochromatin barrier formation.
Different regions of the chromosome have different functions that are typically marked by specific chromatin structures or modification states. We have discussed LCRs that control gene
transcription from very large distances , and that highly compacted heterochromatin (introduced in the chapter Chromosomes) can also spread over large distances .
The existence of these long-range interactions suggests that chromosomes must also contain functional elements that serve to partition chromosomes into domains that can be regulated independently of one another. Over the past several years, the 3C method has been coupled with massively parallel sequencing, resulting in comprehensive interaction maps that probe the three-dimensional architecture of whole genomes. The results indicate that mammalian and Drosophila genomes are organized as a string of TADs that are separated from one another by distinct borders or boundaries (FIGURE1). TADs are characterized by frequent interactions between loci within a domain (e.g., the β-globin genes), but loci within different TADs interact rarely with one another. Thus, TADs might allow for the compartmentalization of chromosomal regions with distinct functions. TADs vary in size, but in mammalian cells they average about 1 Mb. Interestingly, more than half of all mammalian TADs appear conserved between different cell types and even between mouse and human. Other TADs appear to be more dynamic during development. TAD organization is a feature of interphase chromatin, as mitotic chromosomes appear to lack such organization. More recently, similar structures have also been identified in budding and fission yeasts, suggesting that they might be a conserved feature of eukaryotic genomes.
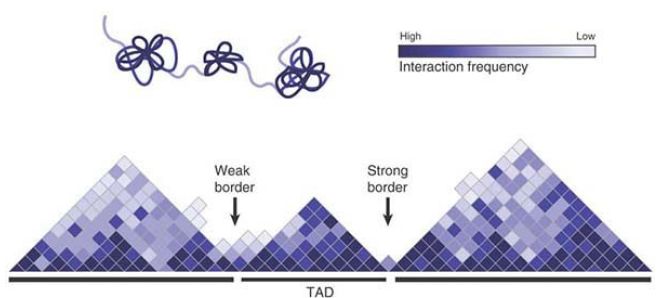
FIGURE 1 Organization of a mammalian genome into strings of TADs. The TADs are defined as regions of the genome that show a high frequency of interactions. TADs are separated by border regions that often contain insulator elements.
The border or boundary elements that separate TADs contain a class of elements called insulators that prevent inter-TAD interactions and block the passage of activating or inactivating effects. Insulators were originally defined as having either or both of two key properties:
-When an insulator is located between an enhancer and a promoter, it prevents the enhancer from activating the promoter. FIGURE 2 shows this enhancer-blocking effect. This activity might explain how the action of an enhancer is limited to a particular promoter despite the ability of enhancers to activate promoters from long distances away (and the ability of enhancers to indiscriminately activate any promoter in the vicinity).
-When an insulator is located between an active gene and heterochromatin, it provides a barrier that protects the gene against the inactivating effect that spreads from the heterochromatin. FIGURE 3 illustrates this barrier effect.
Some insulators possess both of these properties, but others have only one, or the blocking and barrier functions can be separated. Likewise, only some insulators function as borders between TADs, whereas others do not. Although both actions are likely to be mediated by changing chromatin structure, they can involve different effects. In either case, however, the insulator defines a limit for long-range effects. By restricting enhancers so they can act only on specific promoters, and preventing the inadvertent spreading of heterochromatin into active regions, insulators function as elements for increasing the precision of gene regulation.
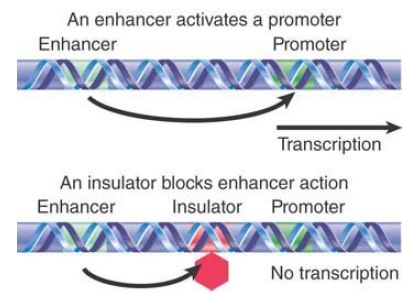
FIGURE 2 .An enhancer activates a promoter in its vicinity but can be blocked from doing so by an insulator located between them.
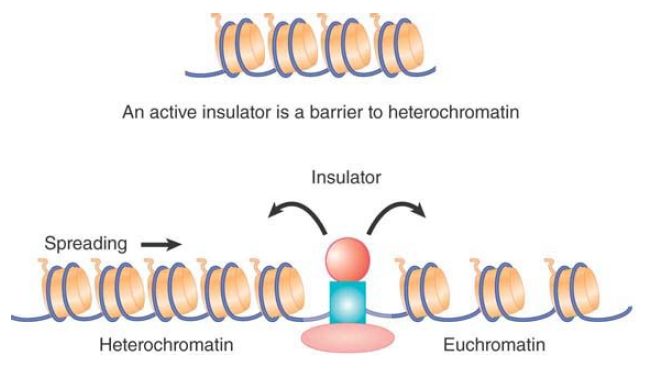
FIGURE 3. Heterochromatin may spread from a center and then block any promoters that it covers. An insulator might be a barrier to propagation of heterochromatin that allows the promoter to remain active.
Insulators were first discovered in the analysis of a region of the Drosophila melanogaster genome shown in FIGURE 4. Two genes for hsp (heat-shock protein) 70 lie within an 18-kb region that constitutes band 87A7. Researchers had noted that when subjected to heat shock, a puff forms at 87A7 in polytene chromosomes, and there is a distinct boundary between the decondensed and condensed regions of the chromosomes. Special structures, called scs and scs′ (specialized chromatin structures), are found at the ends of the band. Each element consists of a region that is highly resistant to degradation by DNase I, flanked on either side by hypersensitive sites that are spaced at about 100 bp.The cleavage pattern at these sites is altered when the genes are turned on by heat shock.
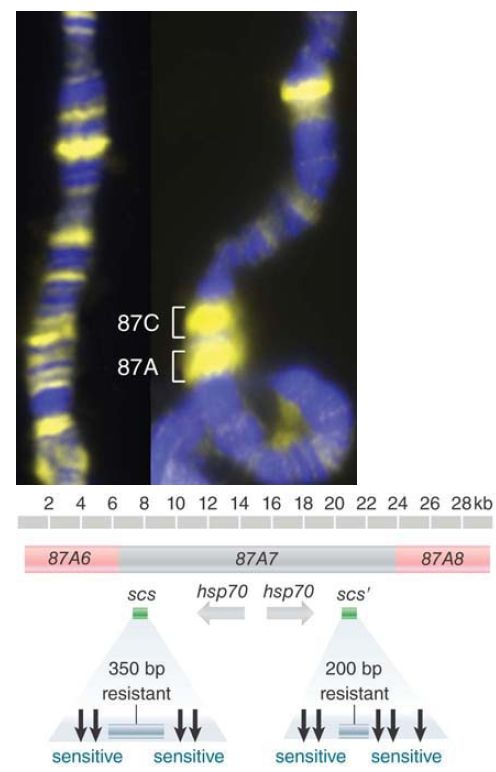
FIGURE 4. The 87A and 87C loci, containing heat-shock genes, expand upon heat shock in Drosophila polytene chromosomes. Specialized chromatin structures that include hypersensitive sites mark the ends of the 87A7 domain and insulate genes between them from the effects of surrounding sequences.
Photo courtesy of Victor G. Corces, Emory University.
The scs elements insulate the hsp70 genes from the effects of surrounding regions (and presumably also protect the surrounding regions from the effects of heat-shock activation at the hsp70 loci).
In the first assay for insulator function, scs elements were tested for their ability to protect a reporter gene from “position effects.” In this experiment, scs elements were placed in constructs flanking the white gene, the gene responsible for producing red pigment in the Drosophila eye, and these constructs were randomly integrated into the fly genome. If the white gene is integrated without scs elements, its expression is subject to position effects; that is, the chromatin context in which the gene is inserted strongly influences whether the gene is transcribed. This can be detected as a variegated color phenotype in the fly eye, as shown in FIGURE 5. However, when scs elements are placed on either side of the white gene, the gene can function anywhere it is placed in the genome—even in sites where it would normally be repressed by context (such as in heterochromatic regions), resulting in uniformly red eyes.
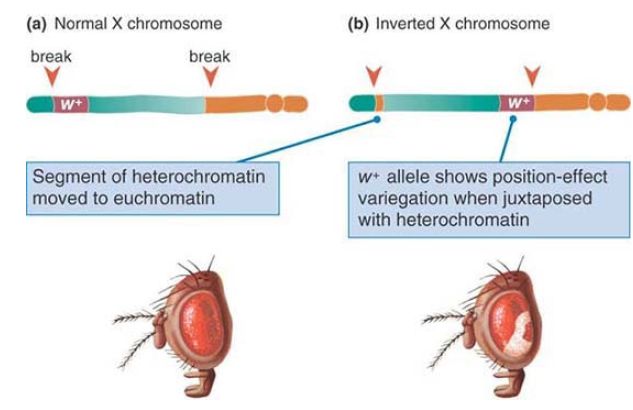
FIGURE 5. Position effects are often observed when an inversion or other chromosome rearrangement repositions a gene normally in euchromatin to a new location in or near heterochromatin. In this example, an inversion in the X chromosome of Drosophila melanogaster repositions the wild-type allele of the white gene near heterochromatin. Differences in expression due to position effects on the w allele are observed as mottled red and white eyes.
The scs and scs′ elements, like many other insulators, do not themselves play positive or negative roles in controlling gene expression, but restrict effects from passing from one region to the next. Unexpectedly, the scs elements themselves are not responsible for controlling the precise boundary between the condensed and decondensed regions at the heat shock puff, but instead serve to prevent regulatory crosstalk between the hsp70 genes and the many other genes in the region. The scs and scs′ elements have different structures, and each appears to have a different basis for its insulator activity. The key +sequence in the scs element is a stretch of 24 bp that binds the product of the zw5 (zeste white 5) gene. The insulator property of scs′ resides in a series of CGATA repeats. The repeats bind a pair of related proteins (encoded by the same gene) called BEAF-32.
BEAF-32 is localized to about 50% of the interbands on polytene chromosomes, suggesting that there are many BEAF-32–dependent insulators in the genome (though BEAF-32 may bind
noninsulators, as well).
Another well-characterized insulator in Drosophila is found in the transposon gypsy. Some experiments that initially defined the behavior of this insulator were based on a series of gypsy insertions into the yellow (y) locus. Different insertions cause loss of y gene function in some tissues, but not in others. The reason is that the y locus is regulated by four enhancers, as shown in FIGURE 6. Wherever gypsy is inserted, it blocks expression of all enhancers that it separates from the promoter, but not those that lie on the other side. The sequence responsible for this effect is an insulator that lies at one end of the transposon. The insulator works irrespective of its orientation of insertion.
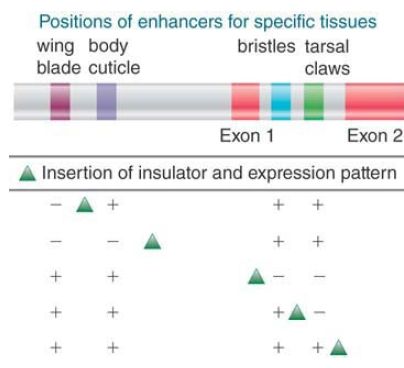
FIGURE 6 The insulator of the gypsy transposon blocks the action of an enhancer when it is placed between the enhancer and the promoter.
The function of the gypsy insulator depends on several proteins, including Su(Hw) (Suppressor of Hairy wing), CP190, mod(mdg4), and dTopors. Mutations in the su (Hw) gene completely abolish insulation; su (Hw) encodes a protein that binds 12 26-bp reiterated sites in the insulator and is necessary for its action.
Su(Hw) has a zinc finger DNA-motif; mapping to polytene chromosomes shows that Su(Hw) is bound to hundreds of sites that include both gypsy insertions and non-gypsy sites. Manipulations show that the strength of the insulator is determined by the number of copies of the binding sequence. CP190 is a centrosomal protein that assists Su(Hw) in binding site recognition.
mod(mdg4) and dTopors have a specific role in the creation of “insulator bodies,” which appear to be clusters of Su(Hw)-bound insulators that can be observed in normal diploid nuclei. Despite the presence of >500 Su(Hw) binding sites in the Drosophila genome, visualization of Su(Hw) or mod(mdg4) shows that they are colocalized at about 25 discrete sites around the nuclear periphery.
This suggests the model of FIGURE7, in which Su(Hw) proteins bound at different sites on DNA are brought together by binding to mod(mdg4). The Su(Hw)/mod(mdg4) complex is localized at the nuclear periphery. The DNA bound to it is organized into loops. An average complex might have 20 such loops. Enhancer–promoter actions can occur only within a loop, and cannot propagate between them. This model is supported by “insulator bypass” experiments, in which placing a pair of insulators between an enhancer and promoter actually eliminates insulator activity—somehow the two insulators cancel out each other. This could be explained by the formation of a minidomain between the duplicated insulator (perhaps too small to create an anchored loop), which would essentially result in what should have been two adjacent loops fused into one. Not all insulators can be bypassed in this way, however; this and other evidence suggests that there are multiple mechanisms for insulator function.
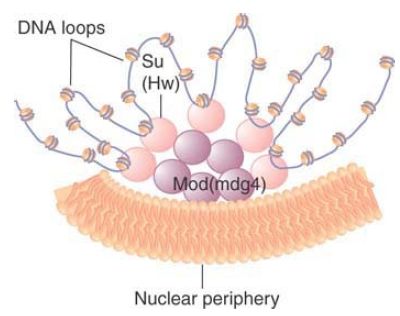
FIGURE 7. Su(Hw)/mod(mdg4) complexes are found in clusters at the nuclear periphery. They can organize DNA into loops that limit enhancer–promoter interactions.
The complexity of insulators and their roles is indicated by the behavior of another Drosophila insulator: the Fab-7 element found in the bithorax locus (BX-C). This locus contains a series of cisacting regulatory elements that control the activities of three homeotic genes (Ubx, abd-A, and Abd-B), which are differentially expressed along the anterior–posterior axis of the Drosophila embryo. The locus also contains at least three insulators that are not interchangeable; Fab-7 is the best studied of these. FIGURE 8 shows the relevant part of the locus. The regulatory elements iab-6 and iab-7 control expression of the adjacent gene Abd-B in successive regions of the embryo (segments A6 and A7). A deletion of Fab-7 causes A6 to develop like A7, resulting in two “A7-like” segments (this is known as a homeotic transformation).
This is a dominant effect, which suggests that iab-7 has taken over control from iab-6. We can interpret this in molecular terms by supposing that Fab-7 provides a boundary that prevents iab-7 from acting when iab-6 is usually active. In fact, in the absence of Fab-7, it appears that iab-6 and iab-7 fuse into a single regulatory domain, which shows different behavior depending on the position along the AP axis. The insulator activity of Fab-7 is also developmentally regulated, with a protein called Elba (Early boundary activity) responsible for Fab-7’s blocking function early in development, but not later in development or in the adult. Fab-7 is also associated with the Drosophila homolog of the CTCF protein, a mammalian insulator-binding protein that shows regulated binding to its targets (see the chapter titled Epigenetics II). In mammalian cells, CTCF is a key component of insulators that form borders between many TADs. Finally, both Fab-7 and a nearby insulator (Fab-8) are known to lie near “anti-insulator elements” (also called promotertargeting sequences or PTS elements), which may allow an enhancer to overcome the blocking effects of an insulator.
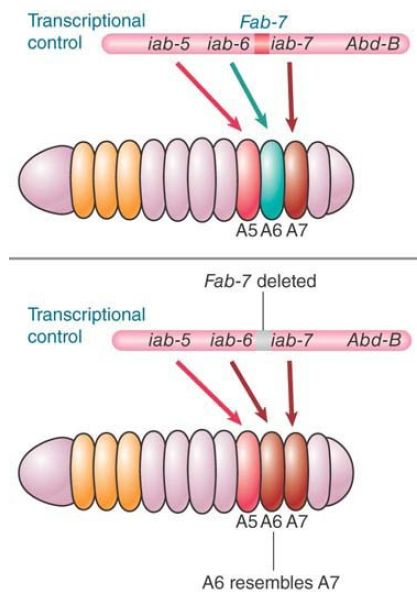
FIGURE 8. Fab-7 is a boundary element that is necessary for the independence of regulatory elements iab-6 and iab-7.
The diversity of insulator behaviors and of the factors responsible for insulator function makes it impossible to propose a single model to explain the behavior of all insulators. Instead, it is clear that the term “insulator” refers to a variety of elements that use a number of distinct mechanisms to achieve similar (but not identical) functions.
Notably, the mechanisms used to block enhancers can be very different from those used to block the spread of heterochromatin. There is also a diversity of proteins that bind to insulator elements, and the general term “architectural proteins” has been used to describe this group of factors. Furthermore, the density of architectural protein binding sites appears to correlate well with different types of insulator activities, with high-density regions corresponding to insulators that function as borders between TAD domains, and lower-density sites regulating intradomain interactions.

|
|
|
|
كيف تعزز نمو الشعر الصحي؟
|
|
|
|
|
|
|
10 فحوصات مهمة يجب القيام بها لسيارتك قبل الصيف
|
|
|
|
|
|
قسم التربية والتعليم ينظّم جلسةً حوارية لملاكه حول تأهيل المعلّمِين الجدد
|
|
|
|
جامعة العميد تحدّد أهداف إقامة حفل التخرّج لطلبتها
|
|
|
|
جامعة العميد تحتفي بتخرّج الدفعة الثانية من طلبة كلّية الطبّ
|
|
|
|
قسم الشؤون الفكريّة يشارك في المؤتمر العلمي الدولي الخامس في النجف الأشرف
|