
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2018
Date: 27-10-2019
Date: 30-9-2019
|
But it doesn't stop here! The diethylamine also reacts with bromoethane - in the same two stages as before. This is where the reaction would start if you reacted a secondary amine with a halogenoalkane.
In the first stage, you get triethylammonium bromide.

There is again the possibility of a reversible reaction between this salt and excess ethylamine in the mixture.
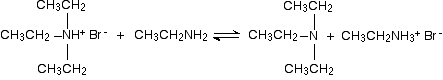
The ethylamine removes a hydrogen ion from the triethylammonium ion to leave a tertiary amine - triethylamine.


|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تنظّم دورةً حول آليّات الذكاء الاصطناعي لملاكاتها
|
|
|