


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-7-2019
Date: 16-5-2017
Date: 26-2-2016
|
As previously seen, aldehydes and ketones react with 2o amines to reversibly form enamines.
Example
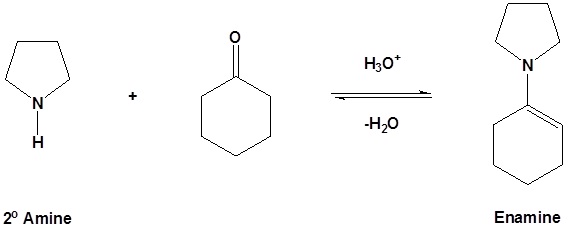
Reversible
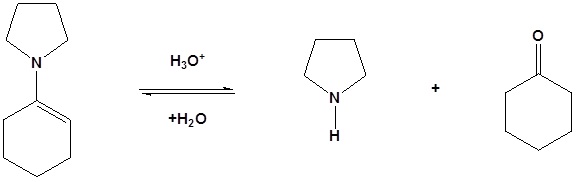
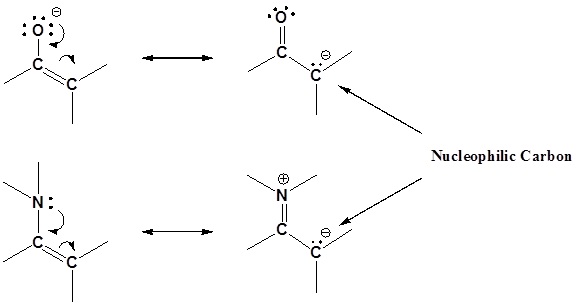
Enamines act as nucleophiles in a fashion similar to enolates. Because of this enamines can be used as synthetic equivalents as enolates in many reactions. This process requires a three steps: 1) Formation of the enamine, 2) Reaction with an eletrophile to form an iminium salt, 3) Hydrolysis of the iminium salt to reform the aldehyde or ketone. Some of the advantages of using an enamine over and enolate are enamines are neutral, easier to prepare, and usually prevent the overreaction problems plagued by enolates. These reactions are generally known as the Stork enamine reaction after Gilbert Stork of Columbia University who originated the work.
Typically we use the following 2o amines for enamine reactions
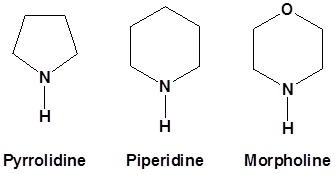



|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تنظّم دورةً حول آليّات الذكاء الاصطناعي لملاكاتها
|
|
|