


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-2-2016
Date: 22-10-2020
Date: 18-9-2019
|
Alternatively, we can transform an alcohol group into sulfonic ester using para-toluene sulfonyl chloride (Ts-Cl) or methanesulfonyl chloride (Ms-Cl), creating what is termed an organic tosylate or mesylate:
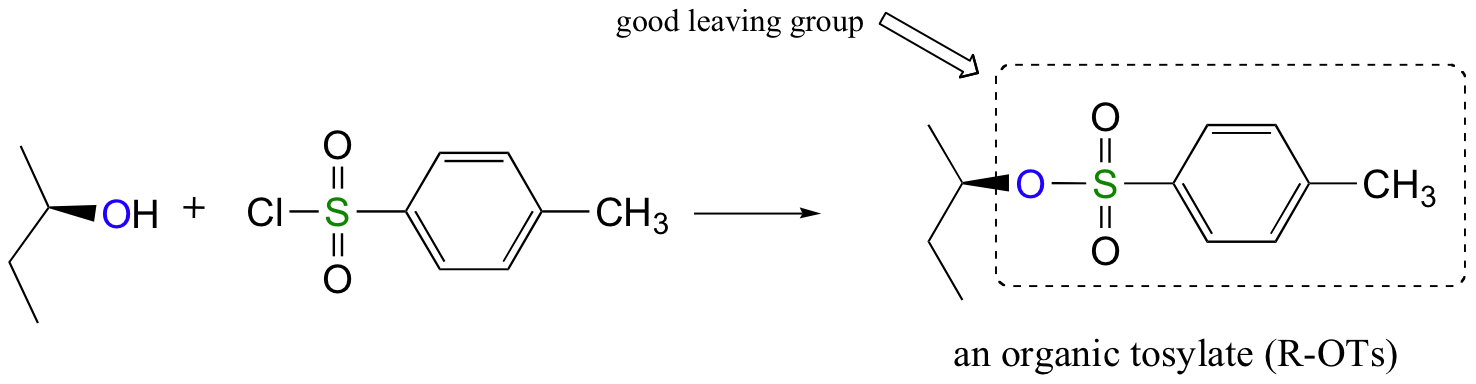
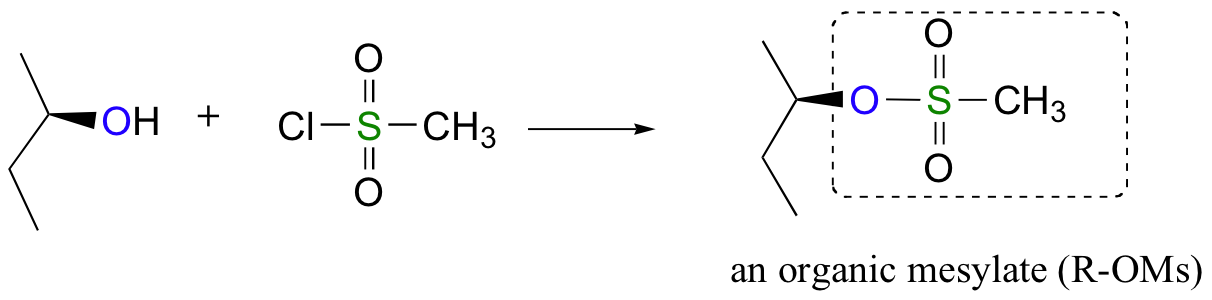
Again, you’ll have a chance to work a mechanism for tosylate and mesylate formation in the chapter 12 problems. Notice, though, that unlike the halogenation reactions above, conversion of an alcohol to a tosylate or mesylate proceeds with retention of configuration at the electrophilic carbon.
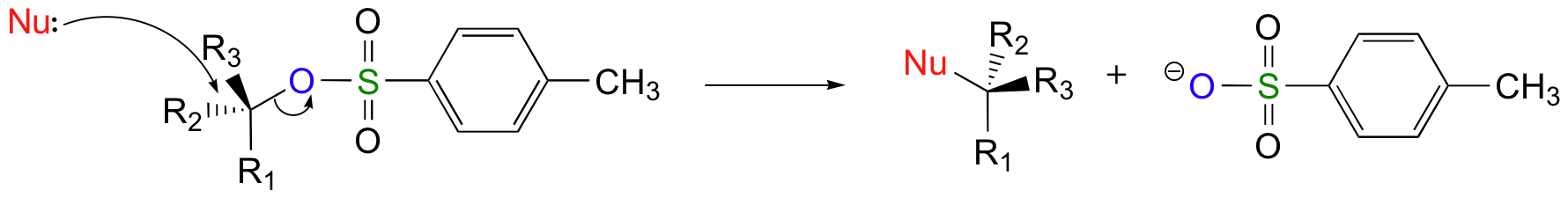
Chlorides, bromides, and tosylate / mesylate groups are excellent leaving groups in nucleophilic substitution reactions, due to resonance delocalization of the developing negative charge on the leaving oxygen.
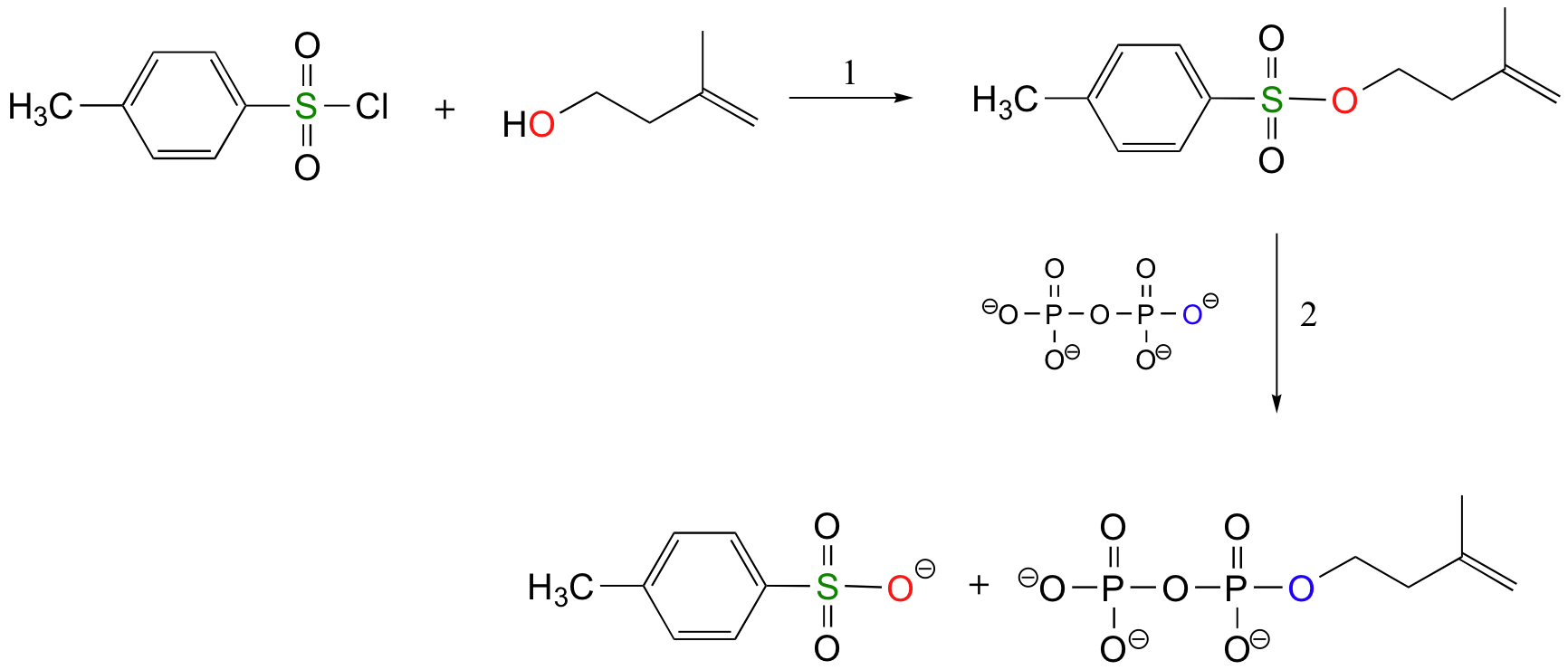
The laboratory synthesis of isopentenyl diphosphate - the 'building block' molecule used by nature for the construction of isoprenoid molecules such as cholesterol and b-carotene - was accomplished by first converting the alcohol into an organic tosylate (step 1), then displacing the tosylate group with an inorganic pyrophosphate nucleophile (step 2).


|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقيم ندوة علمية عن روايات كتاب نهج البلاغة
|
|
|