
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-5-2016
Date: 21-12-2015
Date: 22-9-2019
|
The previous reactions have all involved reagents of the type: Y–NH2, i.e. reactions with a 1º-amino group. Most aldehydes and ketones also react with 2º-amines to give products known as enamines. Two examples of these reactions are presented in the following diagram. It should be noted that, like acetal formation, these are acid-catalyzed reversible reactions in which water is lost. Consequently, enamines are easily converted back to their carbonyl precursors by acid-catalyzed hydrolysis. A mechanism for enamine formation may be seen by pressing the "Show Mechanism" button.
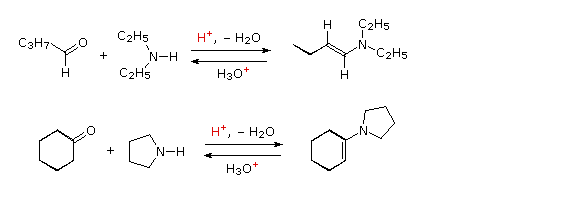
Mechanisim




|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تنظّم دورةً حول آليّات الذكاء الاصطناعي لملاكاتها
|
|
|