
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-10-2019
Date: 26-2-2016
Date: 18-10-2019
|
Examples of Enamine Reactions
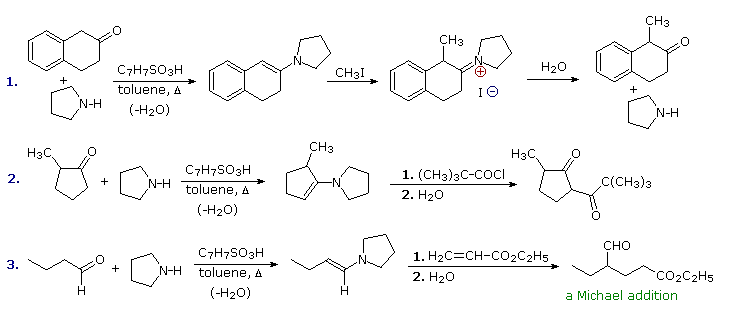
As noted, N-alkylation of enamines is common for aldehydes and some ketones. Michael addition reactions avoid this problem thanks to their reversibility. The third example shows such a reaction, and the "Toggle Mechanism" button displays a possible mechanism. The C-alkylation intermediate is thermodynamically more stable than the N-alkylation species, so it predominates at equilibrium. Both the charges in this intermediate are stabilized by delocalization, and hydrolysis rapidly converts it to the aldehyde-ester product. An interesting alternative is ring closure to a neutral enol ether compound (shown in the blue shaded box) which would also be hydrolyzed to the same product.



|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تنظّم دورةً حول آليّات الذكاء الاصطناعي لملاكاتها
|
|
|