
Aqueous solution chemistry of sulfur, selenium and tellurium
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 464
الجزء والصفحة:
2th ed p 464
 22-3-2017
22-3-2017
 2230
2230
Aqueous solution chemistry of sulfur, selenium and tellurium
As we saw earlier in the chapter, the redox reactions between compounds of S in different oxidation states are often slow, and values of Eo for half-reactions are invariably obtained from thermochemical information or estimated on the basis of observed chemistry. The data in Figure 1.1 illustrate the relative redox properties of some S-, Se- and Te-containing species. Points to note are:
- the greater oxidizing powers of selenate and tellurate than of sulfate;
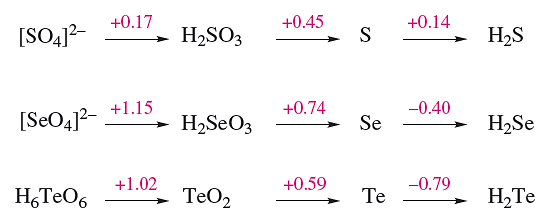
Fig. 1.1 Potential diagrams for sulfur, selenium and tellurium at pH = 0.
- the similarities between the oxidizing powers of sulfate, selenite and tellurite;
- the instabilities in aqueous solution of H2Se and H2Te.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


