
Influence of the Nanocrystal Surface or Interface on the Lattice Parameter
 المؤلف:
C. Br´ echignac P. Houdy M. Lahmani
المؤلف:
C. Br´ echignac P. Houdy M. Lahmani
 المصدر:
Nanomaterials and Nanochemistry
المصدر:
Nanomaterials and Nanochemistry
 الجزء والصفحة:
p50
الجزء والصفحة:
p50
 3-2-2016
3-2-2016
 1388
1388
Influence of the Nanocrystal Surface or Interface on the Lattice Parameter
Several experiments have shown a relationship between the adsorption state of nanocrystal surfaces and the change in the lattice parameter. Hence, for nanocrystals in ceramic powders like BaTiO3 or SrTiO3, the presence of water molecules and OH− ions on the surface causes an increase in the lattice parameter. After desorption of the molecules by a suitable heat treatment, the values for the bulk solid are recovered.
Figure 1 shows how one may adjust the lattice parameter in γ-Fe2O3 by varying the surface energy of the nanocrystal. There are two clearly distinct regimes: first a contraction of the nanocrystal, and then an expansion. The first regime corresponds to a phase in which OH− and H2O are chemisorbed on the surface, and an initial water monolayer is formed, with water vapor pressures below a certain critical value. The second regime, on the other hand, corresponds to the formation of water multilayers by physisorption, and the relaxation of the oxide by strengthening of the bonds between the water layers. The existence of two distinct regimes has also been demonstrated for iron nanoparticles coated with a thin layer of γ-Fe2O3 , and also when oxygen is adsorbed on carbon nanotubes .
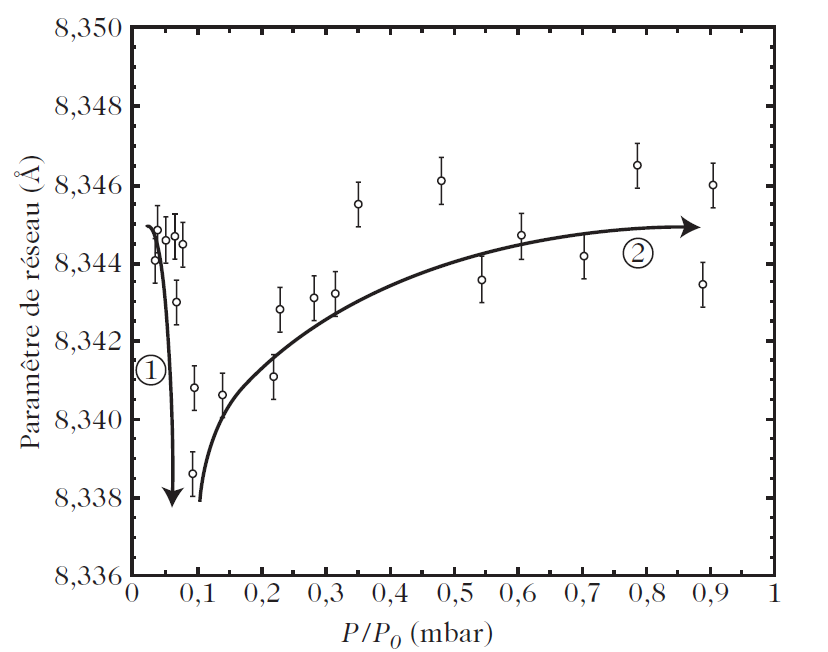
Fig.1. Dependence of the lattice parameter for a nanometric γ-Fe2O3 powder on the state of adsorption of water vapour on the powder. For water vapour pressures below a critical value (around P/P0 = 0.1), the formation of a monolayer appears to compress the oxide, whilst for high pressures, the formation of multilayers of water would appear to relax the oxide by strengthening the bonds between water layers [32]. (1) 630 kJ/mol: chemisorption of OH2− and physisorption of H2O (monolayer on γ-Fe2O3). (2) 45 kJ/mol: physisorption of H2O (multilayer on H2O)
 الاكثر قراءة في كيمياء النانو
الاكثر قراءة في كيمياء النانو
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


