


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 23-12-2015
Date: 16-12-2015
Date:
|
Viral Capsids
The capsids of viruses—the protein part of the virus particle (virion) that surrounds and protects the nucleic acid genome—have been the subject of intense study by biochemists and structural biologists for over 50 years. Capsids are also noteworthy in that they provide one of the few examples in which the detailed properties of a biological system have been predicted successfully from “first principles.” This entry describes the principles on which we understand capsid structure—that is, what we expect capsids to be like and why we expect that, and then describes the ways in which real viruses do or do not follow those expectations.
A gene of double-stranded DNA can encode a protein of only about 1/20 the mass of the gene itself. As a consequence, if a virus is to encode its own capsid (and in almost all cases, viruses do so), it will only be able to make enough protein to produce a useful sized capsid if it can use multiple copies of the protein(s) encoded in its genes. Crick and Watson (1), who made the first concrete proposal for how proteins might be arranged in virus capsids, assumed that the capsids would be made of multiple identical virus-encoded protein subunits. They also made another assumption, based on a prominent property of proteins, namely that proteins are very specific in the interactions they make with other molecules, presumably including other proteins in the structure of a virus capsid. They assumed that the identical protein subunits would be packed into the capsid structure in such a way that they all made the identical set of contacts with their neighbors—so-called equivalent packing. Mathematically, there are only two general ways to satisfy these assumptions when packing asymmetric objects like proteins; these are to arrange the protein subunits with helical symmetry or to arrange them with one of the cubic symmetries. Helical symmetry is easily visualized: The subunits are arranged in a helical array as if they were on successive steps of a spiral staircase. Formally, each subunit is related to the preceding one by a characteristic rotation and a translation in the direction of the helix axis. “Cubic” symmetry refers to a small group of symmetries characterized by having multiple axes of rotational symmetry. These correlate with the five classical Platonic solids, which have these symmetry axes. Besides the cube, from which the entire group of symmetries takes its name, the Platonic solids include the tetrahedron, the octahedron, the dodecahedron, and the icosahedron. Of these, the icosahedron allows (or more properly, arranging protein subunits according to the five-, three-, and twofold rotational symmetry axes of an icosahedron) allows the largest structure to be made with a given size of subunit for any of the group. More to the point, of this group of symmetries, it is icosahedral symmetry that is used by real viruses.
1. Helical Symmetry
Many viruses can be shown to have helical symmetry. The best studied of these is the well-known tobacco mosaic virus (TMV), which has a virion with only one type of protein subunit, present in something over 2000 copies and arranged in a simple helix with 16.33 subunits per turn. There is in principle no limit on how long a helical structure such as this can be, but in TMV it is limited during assembly by the length of the genomic RNA. In the mature virion, the single-stranded RNA genome follows the helix of the proteins and lies in the groove between successive layers of the helix on the inside of the helical tube, three nucleotides per protein subunit. A domain of each subunit closes over this groove after the RNA has entered it during assembly and effectively seals off the RNA from the solvent (2).
Structurally somewhat more complex examples of helically symmetric viruses are the filamentous bacteriophages, such as phage M13. The major protein subunit of these viruses is arranged in what might best be described as a small number of helices running in parallel up a cylindrical tube. The circular single-stranded DNA genome of these viruses is stretched the length of the helical tube. The filamentous phages have small numbers of copies of additional virus-encoded proteins present on the ends of the virions. These have roles in virion assembly and in virus infection (3).
Many enveloped viruses, for example, influenza virus, have their genome—single-stranded RNA in the case of Influenza—wrapped in a helical array with a virus-encoded protein. The resulting flexible nucleoprotein rods are enclosed in the viral envelope, one such structure for each of the eight different genome segments in the case of influenza.
2. Icosahedral Symmetry and Quasi-Equivalent Packing
An icosahedron (Fig. 1) has 20 faces, each an equilateral triangle. Its symmetry is defined by axes of rotational symmetry, each passing through the center of the icosahedron: five-fold axes passing through the corners, three-fold axes passing through the centers of the faces, and twofold axes passing through the centers of the edges. Strictly speaking, an icosahedral virus capsid is not an icosahedron; instead, its protein subunits are related to each other by the symmetry axes of an icosahedron. However, thinking of the protein subunits as lying on the surface of an icosahedron provides a convenient way to visualize and discuss the structure of the capsid, as well as a reasonable approximation to the truth. To make an icosahedrally symmetric capsid, we place three identical protein subunits on each face of the icosahedron, symmetrically arrayed about the center. These subunits will necessarily also find themselves in symmetrical relations with their fellows around all the five-, three-, and twofold axes of the icosahedron, and inspection of a model of such a structure shows that all of the subunits lie in equivalent relationships to their neighbors.
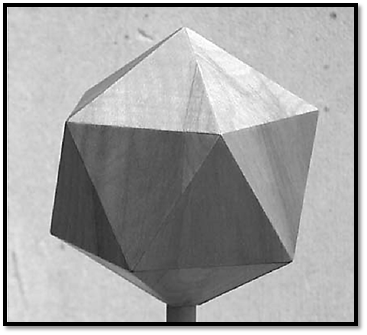
Figure 1. An icosahedron.
Current knowledge of virus structure allows us to see that some small virus capsids—for example, the Parvoviruses—can be adequately described as such 60-subunit icosahedrally symmetric structures. However, it was already clear by about 1960 that the majority of “spherical” viruses are too large and have too many subunits to be so described. Because there is mathematically no way to make a larger structure while preserving strict equivalence in subunit packing, it was not clear that this way of describing capsid structure would be applicable to most viruses. However, Caspar and Klug (4) proposed a clever extension of Crick and Watson's ideas that has provided a framework for understanding and describing the great majority of spherical virus capsids. Caspar and Klug introduced the idea of “quasi-equivalence” in protein subunit packing. In a nutshell, they suggested that if the requirement for equivalence were relaxed—but not abandoned entirely—it would be possible to make much larger capsids with identical protein subunits, containing multiples of 60 subunits.
A simple way to imagine the process of making a larger capsid is to start with a 60-subunit capsid as described above, group the subunits together into the pentamers that surround each corner (fivefold axis) of the icosahedron, and move those pentamers away from the center to a larger radius. Caspar and Klug's insight was that there are certain ways to fill the resulting spaces between the pentamers with hexamers of the same subunit that preserve, at least approximately, the geometrical packing arrangements among all of the subunits in the structure.
A more systematic way to describe this process, shown in Figure 2, is to consider constructing an icosahedron from a piece of paper that has a hexagonal lattice of “protein subunits” printed on it. (Experience has shown, in fact, that the most effective way to understand the concepts described here is to actually take scissors in hand and to construct and examine some capsid models.) The simplest way to make an icosahedron from such a sheet of paper is to cut out 20 of the small triangles in the lattice that each contain three subunits, and join them together into an icosahedron. This icosahedron will have 60 subunits, equivalently packed, and will be identical to the 60-subunit structure described above. It is possible, however, to cut larger triangles out of the paper and use them as the faces of the icosahedron; as long as the corners of these larger triangles fall on the intersection points of the lattice, the resulting icosahedron will be biologically sensible and geometrically acceptable. The figure shows an example of such a larger triangle encompassing four of the small unit triangles. If 20 of these are cut out and assembled into an icosahedron, the structure will have 4 × 60 = 240 protein subunits. The subunits in this case are not all equivalently packed—some are in pentamers and some in hexamers, and there are other subtle differences in their geometrical relationships to their neighbors—but the basic relationships between subunits are nonetheless preserved: They are quasi-equivalent. The faces of this icosahedron are said to be “triangulated” into the four unit triangles, and the structure as a whole is said to have a “triangulation number” (T) of 4. The figure also shows a somewhat more complex way of defining the icosahedral face, in which the edges of the icosahedral face are not parallel to the lattice lines. In the example shown, the area of the face is equal to the area of seven small triangles, and the corresponding icosahedron is given the triangulation number 7. More generally, the triangulation number of any icosahedron can be calculated from the formula T = h2 + hk + k2 , where h and k are the number of steps along the lattice in the h and k directions required to go from one corner of the icosahedral face to another. The “allowed” triangulation numbers derived in this way are a subset of the integers and form an infinite series that starts 1, 3, 4, 7, 9, 12, 13,¼.
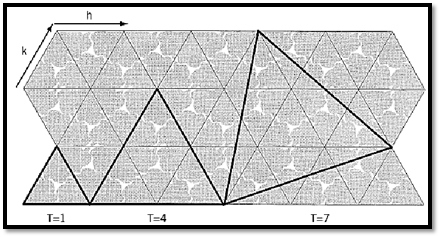
Figure 2. A hexagonal lattice of protein subunits with indications of how they can be fit into a strictly equivalent virus capsid (T = 1) or into two different kinds of quasi-equivalent capsids (T = 4, T = 7). The irregular wedge-shaped objects, of which three are packed into each small triangle, represent protein subunits. The bold triangles show the faces of the capsid icosahedron for the three indicated triangulation numbers. The h and k unit vectors are used to calculate the triangulation number, as described in the text.
3. Icosahedral Virus Capsids in Nature
As the structures of actual virus capsids have been determined in increasing numbers and in increasing detail over the past 30 years, the general predictions of the Caspar and Klug theory have largely been confirmed. At the same time, there are now many examples of “variations on the theme”—features of the structures that are not explicitly predicted by the theory, but are not at odds with its basic concepts. In addition, there are at least two examples of structures that should not have occurred. The definitive test of how the capsid structure is organized is a high-resolution structure by X-ray crystallography; however, it is often possible to get useful information from lower resolution structural measurements. For example, the hexamers and pentamers of the capsid subunit often cluster in such a way that they can be seen by electron microscopy as a distinct morphological unit, called a capsomere. Examining the geometrical relationships among the capsomeres on the surface of a capsid can lead to a good idea of the triangulation number of the capsid.
Parvoviruses, as mentioned above, have 60-subunit T = 1 structures (5). The same is true for the small fX174, bacteriophage, but in this case there are two proteins present in 60 copies each, rather than only one (6). Here we can think of the heterodimer as the basic building block, with 60 copies of that heterodimer arranged with T = 1 icosahedral symmetry.
T = 3 capsids, the first group that requires that quasi-equivalence be invoked, are especially well-populated, with examples from plant, animal, and bacterial viruses. Tomato bushy stunt virus (TBSV), the first of this group to be solved to atomic resolution, conforms quite well to the expectations of the theory, having 180 chemically identical subunits, each of which occupies one of three similar but nonidentical positions in the T = 3 lattice (7). One set of subunits in TBSV, those occupying the “C” position, send an end of their polypeptide chain toward the threefold symmetry axes, where they intertwine with the corresponding parts of the two symmetrically related subunits. This was the first of what are now several examples of capsid proteins interdigitating and intertwining with their neighbors; this is presumed to provide mechanical strength to the capsid. Poliovirus (a Picornavirus) has a structure very similar to that of TBSV, including the positioning of subunits and even the fold of the polypeptide chains within those subunits, but in this case the three quasi-equivalent positions are occupied by three similar but chemically distinct proteins (8). We might imagine that two of the three coding regions encoding these three proteins were the result of gene duplication in an ancestral virus that, like TBSV, had only one capsid protein gene. A slightly different variation on this theme is found in Cauliflower Mosaic virus (a Comovirus), in which the three quasi-equivalent positions are occupied by two proteins, one of which consists of two very similar domains, each occupying one of the three quasi-equivalent positions. These last two examples can be regarded either as slightly noncanonical T = 3 structures or as T = 1 structures made of 60 copies of the heterotrimer (Picornavirus) or heterodimer (Comovirus).
Many capsid structures with larger triangulation numbers have been identified and characterized. Among these are lambda phage, P22 phage, bacteriophage HK97, and others (T = 7), T4 phage and Reovirus (T = 13), Herpesvirus (T = 16), and Adenovirus (T = 25). The largest triangulation number that has been definitively determined is that of the algal virus PBCV1 (T = 169). A particularly instructive example is provided by bacteriophage P2 and its satellite phage P4. The P2 capsid protein assembles into a T = 7 shell; but in the presence of P4, which does not encode its own capsid protein, the P2 capsid protein assembles into a T = 4 structure, just big enough to enclose the P4 genome. This is accomplished through the agency of a P4-encoded protein that associates transiently with the P2 capsid protein during assembly and directs it into the T = 4 geometry (9).
Although these large viruses all fit into the general picture envisioned by Caspar and Klug, they are replete with exceptions and extensions to the original picture, and these variations all expand our view of how capsids can be constructed. For bacteriophage T4 and Adenovirus, the proteins that make the pentamers at the five-fold symmetric corners of the capsid are encoded by a different gene from the proteins at the six-fold positions in the remainder of the capsid. This is analogous to the Picornavirus example above and presumably reduces the amount of conformational or bonding versatility demanded of any one protein, at the relatively minor expense of encoding an additional protein. In T4, the major component of the capsid, which occupies the hexamer positions, is, as expected, a hexamer, but in Adenovirus these positions are occupied by trimers of the “hexon protein” (10). However, the hexon protein is organized into two similarly folded domains, and the structure of the trimeric hexon is very close to a sixfold symmetric arrangement of those domains.
Some viruses—particularly but not exclusively some of the double-stranded DNA phages—have prolate capsids, which have a standard icosahedral arrangement of subunits, except that the shell is elongated along one of the fivefold axes of symmetry and an extra band of hexamers is inserted around the equator. Thus bacteriophage T4 has an elongated T = 13 capsid, and bacteriophage f29 has an elongated T = 4 capsid. These structures pose interesting questions with regard to how their length is specified and accurately achieved, but they cause no serious problems for the idea of quasi-equivalent packing.
Most viruses have other capsid subunits in addition to the main, icosahedrally packed, subunit protein. In some cases, these are present in equimolar amounts with the main subunit and packed with the same symmetry. Thus phages l and T4 have well-studied examples of such “decoration proteins”; Herpesvirus has a protein clustered as trimers that fits this description, but is systematically absent from positions immediately surrounding the pentamers. Proteins in this category are known in some cases to provide additional strength and stability to the capsid. Structurally, they can be regarded simply as additional domains of the main capsid protein, albeit ones that are encoded by separate genes and generally join the structure at a different time.
Many capsids also have “minor” proteins that are not arrayed with icosahedral symmetry. The portal protein of the double-stranded DNA phages, for example, forms a grommet-like 12-subunit oligomer that replaces a pentamer at one of the five-fold corners of the icosahedral shell and provides an attachment site for the six-fold symmetric helical tail (11). Adenovirus has a trimeric spike extending out from the shell along each of its five-fold symmetry axes.
The first radical deviation from the expectations of the Caspar–Klug ideas was found in the virion of Papovavirus SV40. The capsomeres of SV40 visible by electron microscopy are arranged as expected for a T = 7 structure. However, all of the capsomeres are pentamers of the VP1 subunit (12) ,including the 60 capsomeres situated at positions of sixfold local symmetry, which would be expected to be hexamers in order to interact quasi-equivalently with their environment. The subunits adapt to this extraordinary state of affairs by having the part of the polypeptide chain that contacts the neighboring capsomere located on a flexible arm corresponding to the C-terminus of the subunit.
The C-terminal arm leaves its home subunit at dramatically different angles in different cases, allowing it to interact with its neighbor in essentially the same way in each case. This it does by invading the structure of the neighbor and forming one strand of a b-sheet structure in that subunit. This might be regarded as a form of quasi-equivalent interaction, but of a sort requiring a much more radical subunit flexibility than envisioned by Caspar and Klug.
Another surprising arrangement of subunits is found in fungal virus L-A and bacteriophage f6. These capsids have 120 identical protein subunits, not one of the “allowed” numbers. These are T = 1 structures, built of 60 asymmetric homodimers. The unexpected feature of this arrangement is that the two chemically identical subunits occupy nonequivalent positions. Nonetheless, these capsids, as for those of SV40, evidently function well enough to have survived natural selection (ie, very well indeed), and an understanding of their structures enlarges our view of the capabilities of proteins.
References
1. F. Crick and J. D. Watson (1956) Nature 177, 473–475.
2. J. N. Champness et al. (1976) Nature 259, 20–24.
3. L. Makowski and M. Russel (1997) In Structural Biology of Viruses (W. Chiu, R. M. Burnett, and R. L. Garcea, eds.), Oxford University Press, New York, pp. 352–380.
4. D. Caspar and A. Klug (1962) Cold Spring Harbor Symp. Quant. Biol. 27, 1–24.
5. J. Tsao et al. (1991) Science 251, 1456–1464.
6. R. McKenna, L. Ilag, and M. Rossmann (1994) J. Mol. Biol. 237, 517–543.
7. A. J. Olson, G. Bricogne, and S. C. Harrison (1983) J. Mol. Biol. 171, 61–93.
8. J. Hogle, M. Chow, and D. Filman (1985) Science 229, 1358–1365.
9. O. Marvik et al. (1995) J. Mol. Biol. 245, 59–75.
10. R. M. Burnett (1997) In Structural Biology of Viruses (W. Chiu, R. M. Burnett, and R. L. Garcea, eds.), Oxford University Press, New York, pp. 209–238.
11. C. Basinet and J. King (1985) Annu. Rev. Microbiol. 39, 109–129.
12. R. L. Garcea and R. C. Liddington (1997) In Structural Biology of Viruses (W. Chiu, R. M. Burnett, and R. L. Garcea, eds.), Oxford University Press, New York, pp. 187–208.

|
|
|
|
صنع الذكريات والتفكير يدمر الدماغ.. دراسة تشرح السبب
|
|
|
|
|
|
|
الصين.. عودة كاسحتي الجليد إلى شنغهاي بعد انتهاء بعثة استكشافية إلى القطب الجنوبي
|
|
|
|
|
|
العتبة العباسية تختتم فعاليات حفل سنّ التكليف الشرعي المركزي لطالبات المدارس في كربلاء
|
|
|
|
العتبة العباسية تكرم المساهمين بنجاح حفل التكليف الشرعي للطالبات
|
|
|
|
ضمن فعاليات حفل التكليف الشرعي.. السيد الصافي يلتقط صورة جماعية مع الفتيات المكلفات
|
|
|
|
حفل الورود الفاطمية يشهد عرضًا مسرحيًّا حول أهمية التكليف
|